
 |
Original Research (Peer-reviewed)
Rajic A, Dewey CE, Deckert AE, et al. Production of PRRSV-negative pigs commingled from multiple, vaccinated, serologically stable, PRRSV-positive breeding herds. J Swine Health Prod. 2001;9(4):179-184. Also available as a PDF.
Objectives: To determine whether PRRSV-negative pigs could be produced from multiple PRRSV-positive herds and to investigate the serological stability of these herds and the impact of sow vaccination.
Methods: Piglets originating from non-vaccinated and vaccinated herds were weaned at 8 to 14 days and housed separately in an off-site nursery for 90 days. Pigs were tested by PRRS IDEXX ELISA at weaning and at 30, 60, and 90 days of age. A subset of pigs was tested using PRRS reverse transcription-polymerase chain reaction (RT-PCR) and restriction fragment length polymorphism (RFLP) assays. Serological stability of breeding herds was assessed by PRRS IDEXX ELISA.
Results: The five vaccinated herds were serologically stable. Piglets from these herds became seronegative after weaning and remained PRRSV negative up to 90 days of age.
Pigs from two of the five non-vaccinated herds were PRRSV negative. One of these herds was serologically stable. Piglets from the other three non-vaccinated herds were PRRSV positive at weaning, and the number of seropositive piglets and their S:P values increased to 90 days of age. Two of these herds were not serologically stable. After vaccination of open sows and gilts, PRRSV-negative pigs were produced from one of these two herds.
Implications: Piglets weaned at 8 to 14 days from multiple, serologically stable sow herds and commingled were PRRSV negative at 90 days of age. Vaccinating gilts twice prior to breeding and sows at weaning may increase the stability of breeding herds. Results must be validated in large commercial operations.
Keywords : swine, PRRS,
serologically stable, vaccination, early weaning
: swine, PRRS,
serologically stable, vaccination, early weaning
Received: June 17, 1999
Accepted: March 22, 2001
Porcine reproductive and respiratory \syndrome (PRRS) has become wide\spread in almost all pig-producing countries worldwide. Many countries require that imported pigs come from PRRSV-negative areas or herds. Thus, the production of PRRSV-negative pigs has become important to the pork industry for the export of valuable breeding stock and the establishment of new PRRSV-negative nucleus herds. A number of management strategies have been used in the industry to control or eradicate PRRSV virus in herds,2-3,9-11,14-16,17 but attempts have not been consistently successful.4,6,10 The success of any control or eradication strategy requires correct identification of herd-specific viral patterns and susceptible populations on the farm.1,7-8,9,13 If there is active virus circulation in the breeding herd, piglets are likely to be infected before weaning. If the breeding herd is stable, infection of piglets prior to weaning is less likely.7,12 Serological stability of breeding herds is traditionally assessed using PRRS enzyme-linked immunosorbent assay (ELISA) S:P descriptive statistics.5,12 Serology may not be adequate to define the absence of vertical transmission of PRRSV virus in the breeding herd, and it may be necessary to use molecular techniques such as PCR to determine the pattern of viral infection within the farm.13,15
The main objective of this study was to repeatedly produce PRRS-negative pigs from a nursery supplied by multiple PRRS-positive herds, and to maintain PRRS-negative status in the pigs to 90 days of age. In addition, we investigated serological stability of these herds and the impact of sow vaccination.
Three trials were performed using the following protocol. A PRRS IDEXX ELISA (HerdCheck(R), IDEXX Laboratories Inc., Westbrook, Maine) was performed on 15 to 30 randomly selected sows and gilts in each breeding herd to assess the serological status of the herd according to criteria for a serologically stable breeding herd. Piglets were weaned at 8 to 14 days of age and commingled in an off-site nursery, with pigs from vaccinated and non-vaccinated herds housed separately. All pigs were serologically tested four times (PRRS IDEXX ELISA) between weaning and 90 days of age. A subset of pigs was tested with PRRS reverse transcription-polymerase chain reaction (RT-PCR) and restriction fragment length polymorphism (RFLP) assays.
Three trials were conducted in two rooms with separate ventilation, manure handling, and personnel. Pigs from vaccinated and non-vaccinated herds were housed in separate rooms. Management was all in-all out by room, except in Trial 2 when piglets were weaned on three occasions and filling took 3 weeks to accomplish. Except in Trial 1, pigs from different herds were separated by pen.
The health status of the pigs was monitored by the nursery manager on a daily basis and by a veterinarian at the time of blood sampling. The manager recorded health changes and therapeutic treatments.
Ten herds (seven from Ontario and three from Quebec) were selected by the owner of the off-site nursery according to the genetic needs of his export company and the willingness of producers to participate in the study (Table 1). All herds were PRRS seropositive, either by infection with a field strain (Herds 6 to 10) or by vaccination (Herds 1 to 5) with Ingelvac RespPRRS(TM) (Boehringer-Ingelheim, St. Joseph, Missouri). Clinical PRRS had occurred in vaccinated Herds 1, 4, and 5, while vaccinated Herds 2 and 3 reported PRRS-negative status before vaccination was initiated. The five non-vaccinated herds reported PRRS-seropositive status without clinical PRRS. In all vaccinated herds, gilts were vaccinated twice at a 2-week interval, and sows were vaccinated prior to breeding, except in Herd 5, where sows were vaccinated in mid-gestation. All herds had used the same vaccination protocols for more than 3 years (Table 1).
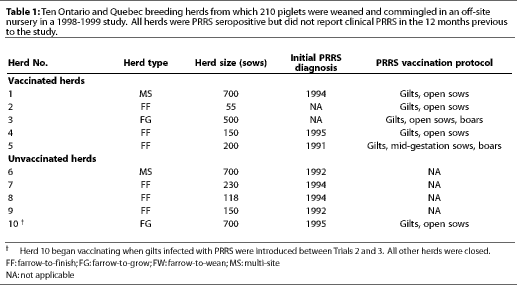
Herds 1 through 9 were closed to new additions. Herd 10 was an open, non-vaccinated herd that became re-infected with PRRSV through the introduction of infected gilts which were housed in the same barn with the PRRS-seronegative, first trimester, gestating gilts. When the owner became aware of their PRRS status, between Trials 2 and 3, gilts and sows in this herd were vaccinated prior to breeding (Ingelvac RespPRRS(TM)).
Fifty-three dams were selected by ten participating producers. Approximately one third to one half of the pigs from the 53 selected litters were sent to an off-site nursery, while the other piglets remained on the farm of origin. Participation of herds, number of litters and piglets per herd, weaning age, and source and number of piglets in Trials 1, 2, and 3 are provided in Tables 2 and 3.
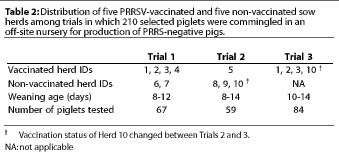
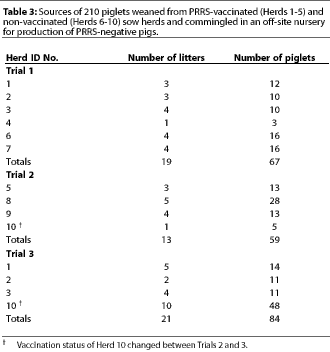
For non-vaccinated herds, sample size was calculated to provide a 95% level of confidence for estimating a seroprevalence of at least 30% +/- 15%.19 For vaccinated herds, sample size was calculated to provide a 95% level of confidence for estimating a seroprevalence of at least 60% +/- 20%.19 A cross-sectional serological profile of 15 to 30 randomly selected breeding animals per herd was performed, with the sample size adjusted to herd size.19 This profile included gilts (prior to breeding and vaccination), and gestating and lactating sows over a full range of parities and trimesters of gestation. Herds that participated in only one trial were sampled once (Herds 4, 5, 6, 7, 8, and 9), while herds that participated in two trials, except Herd 10, were sampled on two occasions almost a year apart (Herds 1, 2, and 3). Herd 10 was sampled only once, prior to Trial 2, due to logistical constraints. The 210 piglets that were moved to the off-site nursery were sampled four times: at weaning, and at 30, 60, and 90 days of age. At least three sera representing piglets from each herd were randomly selected for RT-PCR testing and were representative of the off-site nursery. Sera were also tested from pigs that showed an increase in PRRS ELISA S:P values with successive tests.
Serology was performed using PRRS IDEXX ELISA (HerdCheck(R), IDEXX Laboratories Inc., Westbrook, Maine). An S:P of >=0.4 was considered seropositive. All samples from a herd and from a particular pig were tested on the same day. A diagnostic RT-PCR was conducted on selected individual sera to confirm and clarify serological findings in piglets. An RFLP assay was used to type the virus in the PCR-positive sera.23 This test provides a numerical, three-digit code that describes open reading frame RFLP patterns for restriction enzymes Mlu I, Hinc II, and Sac II, in that order. The same laboratory personnel at the Animal Health Laboratory, Laboratory Services Division, University of Guelph, conducted PRRS IDEXX ELISA, PCR, and RFLP assays.
The following definition was modified from Dee.12 A vaccinated herd was considered serologically stable if the PRRS ELISA S:P was <2.0 for at least 90.0% of breeding herd serum samples. A non-vaccinated herd was considered serologically stable if the PRRS ELISA S:P was <1.0 for at least 90% of breeding herd samples, and if less than 10.0% of samples had an S:P >2.0.
Descriptive statistics were performed using the xChek(TM) IDEXX Global Animal Health Monitoring software (version 2.0, 7/1997).
No deaths were recorded. Two piglets were euthanised (Trials 1 and 3) because of physical injuries. Two pigs infected with Staphylococcus hyicus were treated with parenteral antibiotics (Trial 2). No other clinical illnesses were recorded.
All 35 piglets from the vaccinated herds (Herds 1, 2, 3, and 4) were seronegative at 60 to 90 days of age, and the ten sera tested by RT-PCR were PRRSV negative.
Sixteen of the 32 piglets from the two non-vaccinated herds
(Herds 6 and 7) were seropositive at weaning. The number of seropositive
piglets and their S:P values increased successively in the 30-day
and 60-day tests. Five of ten sera tested by RT-PCR were PRRSV
positive. In three piglets from Herd 6 and one from Herd 7, a
PRRSV field strain with an RFLP cut-pattern of 1-1-2 was identified
when positive samples were tested by RT-PCR RFLP. An intermediate
strain with a cut-pattern of 2-1-2 was identified in one piglet
from
Herd 6.
All 13 piglets from the vaccinated herd (Herd 5) were seronegative at 60 and 90 days of age, and the three sera tested by RT-PCR were PRRSV negative.
Ten of the 28 piglets from the non-vaccinated Herd 8 were seropositive at weaning. All 28 piglets were seronegative at 30 days of age and remained seronegative to the end of the trial. The 13 pigs from non-vaccinated Herd 9 were seronegative throughout the trial. All seven sera tested by RT-PCR from Herds 8 and 9 were PRRSV negative.
The five piglets from non-vaccinated Herd 10 were seropositive at weaning and had increasing PRRS ELISA S:P values (up to 3.1) in the 30-day and 60-day tests. All five sera tested by RT-PCR were PRRSV positive. Intermediate strains with cut patterns of 2-1-2 and 1-5-2 were identified using RT-PCR RFLP.
All 84 piglets from vaccinated Herds 1, 2, and 3, and the recently vaccinated Herd 10, were seronegative at 60 and 90 days of age. All 12 sera tested by RT-PCR were PRRSV negative.
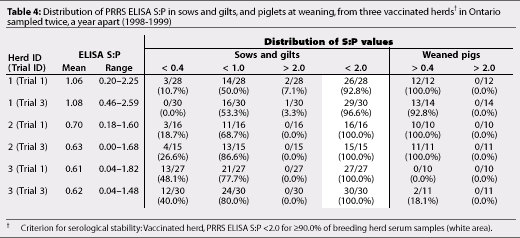
Vaccinated Herds 1 to 5 and non-vaccinated Herds 6 and 8 were serologically stable breeding herds according to the criteria used to define serological stability (Tables 4 and 5). Non-vaccinated Herds 7, 9, and 10 did not meet these criteria (Table 5).
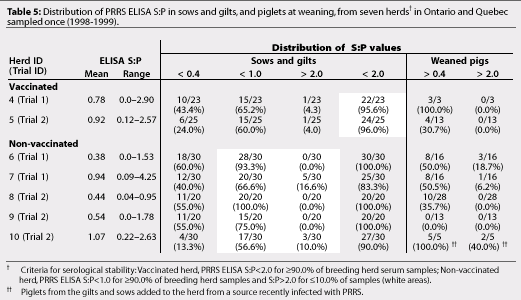
Other studies reported production of PRRSV-negative pigs from PRRSV-positive, non-vaccinated herds by use of the Isowean system, by deriving piglets by hysterectomy, and by onsite or offsite early weaning.3,15,17 In our study, PRRSV-negative pigs were produced from two of five non-vaccinated herds, and from five of five vaccinated, serologically stable herds. The number of seropositive piglets declined gradually in the 30- and 60-day samples from vaccinated herds, indicating a loss of passive antibody.6,18,26
Other researchers were not successful in consistently eliminating PRRSV by early weaning4,6, possibly because of transplacental transmission of the virus from dams to piglets during gestation, or shedding of PRRSV in milk or colostrum.20,22 In our study, the number of PRRSV-positive piglets in non-vaccinated Herds 6 and 7 increased between the 30- and 60-day tests. Piglets from Herd 6 that were PRRSV positive by RT-PCR at 8 to 10 days of age were probably vertically infected. The PRRSV was not detected in piglets from Herd 7 at weaning, but by 30 days of age, one pig was PCR-test positive and two had S:P values >2.0. Detection of the same field strain (RFLP cut pattern 1-1-2) in piglets from both herds suggests that piglets from Herd 7 were infected after being housed with PRRSV-positive piglets from Herd 6. However, because of the unstable serological profile of breeding Herd 7, with PRRS ELISA S:P values up to 4.25, the possibility of infection prior to weaning cannot be excluded.
If the breeding herd is stable, piglets are less likely to be infected before weaning.12 We were able to produce PRRSV-negative pigs from all five serologically stable vaccinated herds. Our findings support Dee's12 definition of serological stability in vaccinated herds. Dean5 suggested that stable vaccinated herds had an average PRRS ELISA S:P <1.0, while unstable herds had an average PRRS ELISA S:P >1.0, and defined the region of stability as the mean S:P across negative farms, +/-3 SD. In our study, mean PRRS ELISA S:P was <1.0 in four of the five vaccinated herds, but in Herd 1, mean PRRS ELISA S:P was 1.06 in Trial 1 and 1.08 in Trial 3. Consistent production of PRRS-negative pigs in Trials 1 and 3, and confirmation of their PRRSV-negative status by RT-PCR assay, suggested that Herd 1 was correctly considered a serologically stable herd.
Herds 6 and 8 were serologically stable non-vaccinated herds according to the suggested criteria. However, detection by RT-PCR assay of PRRSV-positive piglets from Herd 6 at weaning indicated active virus transmission in the lactating population, and herd instability. This suggests that serological tests alone may not be adequate to define the absence of vertical transmission in a breeding herd,13 although serological assessment using suggested criteria may be a good indicator of herd stability. Suggested cut-off values for vaccinated and non-vaccinated, serologically stable breeding herds cannot be considered standardised, particularly in non-vaccinated herds. Until a standardised definition of a serologically stable herd has been validated, the term 'serologically stable' remains relatively subjective.
The vaccination status of Herd 10 changed during the study. This herd participated in Trials 2 and 3 with some interesting results. In Trial 2, piglets from Herd 10 (unstable, non-vaccinated herd) were housed for 75 to 80 days in different pens in the same room as PRRS-negative pigs produced from Herds 8 and 9 (stable, non-vaccinated herds). Piglets from Herd 10 were seropositive at 30 days of age and showed increasing PRRS ELISA S:P values, up to 3.1, to the end of trial. Pigs from the stable, non-vaccinated herds remained seronegative during the trial, suggesting that the importance of aerosol transmission of PRRSV might be overestimated.25 In Trial 3, PRRSV-negative pigs were produced from Herd 10 after gilts and sows had been vaccinated prior to breeding. As the post-vaccination serologic profile from Herd 10 was unavailable, we were unable to evaluate the effect of vaccination on the serological stability of the breeding herd.
In our study, vaccinated herds were more likely to be serologically stable than non-vaccinated herds. Vaccinating gilts twice prior to breeding and sows at weaning for more than 3 years appeared to produce serological stability. In Herds 1, 2, and 3, similar distributions of PRRS ELISA S:P values were seen in sows and piglets in two tests almost a year apart (Table 4). Pigs negative for PRRSV were produced repeatedly from these relatively small, closed, serologically stable herds (<700 sows) with good biosecurity. However, Sanford21 also reported that pigs seronegative for PRRS at the end of the nursery phase were produced from three large commercial production systems (>1000 sows) that were representative of large commercial swine operations in North America. In these units, sows were vaccinated routinely for more than 3 years and breeding herds were serologically stable, ie, PRRS ELISA S:P was <2.0 in at least 90.0% of breeding herd samples.
Our protocol was validated in a small, off-site nursery designed for the specialised production of high health breeding stock for export and the establishment of new nucleus herds, and our results may be biased due to the small sample size on a herd level and the selection procedure of herds and dams within herds. These results may not apply to large herds (>1000 sows) with a rapid turnover of breeding animals, commingling from several sow herds, and less stringent biosecurity measures.
We wish to acknowledge Donaldson Livestock International; Dr. Andre Broes, CDPQ, Sainte-Foy, Quebec; Dr. Susy Carman (Virology Department) and Hazel Alexander (Molecular Biology Department), Animal Health Laboratory, Laboratory Service Division, University of Guelph, Ontario; Dr. Ernest Sanford, Boehringer-Ingelheim, Canada; and the participating swine producers.
Financial support was provided by Ontario Pork, the Ontario Ministry of Agriculture and Rural Affairs, Boehringer- Ingelheim, Canada, and Agriculture and Agri-Food Canada through the CanAdapt program administered by the Ontario Adaptation Council.
1. AASP Subcommittee on PRRS. Laboratory diagnosis of porcine reproductive and respiratory syndrome (PRRS) virus infection. Swine Health Prod. 1996;4:33-35.
2. AASP Subcommittee on PRRS. Control of porcine reproductive and respiratory syndrome (PRRS) virus. Swine Health Prod. 1996;4:95-97.
3. Bruna G, Cabeza de Vaca S, Joo HS, Pijoan C. Comparison of techniques for controlling the spread of PRRSV in large swine herd. Swine Health Prod. 1997;5:59-65.
4. Clark LK, Hill MA, Kniffen TS, VanAlstine W, Stevenson G, Meyer KB, Wu CC, Scheidt AB, Knox K, Albregts S. An evaluation of the components of medicated early weaning. Swine Health Prod. 1994;2:5-11.
6. Dee SA, Morrison RM, Joo HS. Eradicating porcine reproductive and respiratory syndrome (PRRS) virus using two-site production and nursery depopulation. Swine Health Prod. 1993;1:20-23.
7. Dee SA, Joo HS, Henry S, Tokach L, Park BK, Molitor TW, Pijoan C. Detecting subpopulations after PRRS virus infection in large breeding herds using multiple serologic tests. Swine Health Prod. 1996;4:181-184.
8. Dee SA, Joo HS. PRRS serology: A critical component of the diagnostic workup. Swine Health Prod. 1996;4:100-101.
9. Dee SA, Joo HS. Strategies to control PRRS: a summary of field and research experiences. Vet Micro. 1997;55:347-353.
10. Dee SA, Joo HS, Park BK, Molitor T W, Bruna G. Attempted elimination of porcine reproductive and respiratory syndrome virus from a seedstock farm by vaccination of the breeding herd and nursery depopulation. Vet Rec. 1998;142:569-572.
11. Dee SA, Philips R. Using vaccination and unidirectional pig flow to control PRRSV transmission. Swine Health Prod. 1998;6:21-25.
13. Dee SA, Philips RE. Use of polymerase chain reaction (PCR) to detect vertical transmission of porcine reproductive and respiratory syndrome virus (PRRSV) in piglets from gilt litters. Swine Health Prod. 1999;7:237-239.
14. Dee SA, Molitor TW, Rossow, D. Epidemiological and diagnostic observations following the elimination of porcine reproductive and respiratory syndrome virus from a breeding herd of pigs by the test and removal protocol. Vet Rec. 2000;146:211-213.
15. Donadeu M, Arias M, Gomez-Tejedor C, Aguero M, Romero LJ, Christianson WT, Sanchez-Vizcaino JM. Using polymerase chain reaction to obtain PRRSV-free piglets from endemically infected herds. Swine Health Prod. 1999;7:255-261.
16. Fangman TJ, Tubbs RC, Henningsen-Dyer K. Influence of weaning site, weaning age, and viral exposure on production performance in early-weaned nursery pigs. Swine Health Prod. 1996;4:223-229.
18. Houben S, Van Reeth K, Pensaert MB. Pattern of infection
with the porcine reproductive and respiratory syndrome virus on
swine farms in Belgium.
J Vet Med B. 1995;42:209-215
19. Martin SW, Meek AH, Willeberg P. Veterinary Epidemiology-principles and methods. Ames, Iowa: Iowa State University Press. 1987;22-42.
20. Prieto C, Suarez P, Simmaro I, Garcia C, Fernandez A, Castro JM.Transplacental infection following exposure of gilts to porcine reproductive and respiratory syndrome virus at the onset of gestation. Vet Micro. 1997;57:301-311.
23. Wesley RD, Mengeling WL, Lager KM, Clouser DF, Landgraf JG, Frey ML. Differentiation of a porcine reproductive and respiratory syndrome virus vaccine strain from North American field strains by restriction fragment length polymorphism analysis of ORF 5. J Vet Diagn Invest. 1998;10:140-144.
24. Wesley RD, Mengeling WL, Lager KM, Vorwald AC, Roof MB. Evidence for divergence of restriction fragment length polymorphism patterns following in vivo replication of porcine reproductive and respiratory syndrome virus. Am J Vet Res. 1999;60:463-467.
25. Wills RW, Zimmermann JJ, Swenson SL, Yoon KJ, Hill HT, Bundy DS, McGinley, MJ. Transmiss-ion of PRRSV by direct, close, or indirect contact. Swine Health Prod. 1997;5:213-218.
26. Yoon KJ, Zimmerman JJ, Swenson SL, McGinley MJ, Eernisse KA, Brevik A, Rhinehart LL, Frey ML, Hill TH, Platt KB. Characterization of the humoral immune response to porcine reproductive and respiratory syndrome (PRRS) virus infection. J Vet Diagn Invest. 1995;7:305-312.
5. Dean A, Deen J. A comparison of serologic profiles to define PRRS "stability" in herds using a commercial vaccine. Proc 15th IPVS Cong. 1998;267.
12. Dee SA. A protocol to define breeding herd stability and classify farms according to PRRS status to identify potential intervention strategies: A summary of 200 farms. Proc AASP Ann Meet. DesMoines,Iowa. 1998;409-411.
17. Gramer ML, Christianson WT, Harris DL. Producing PRRS negative pigs from PRRS positive sows. Proc AASP Ann Meet. New Orleans, Louisiana. 1999;413-416.
21. Sanford SE. Production of PRRS seronegative pigs from vaccinated stable PRRS - positive sow herds. Proc 16th IPVS Cong. 2000;589.
22. Wagstrom EA, Chang CC, Yoon KJ, Zimmerman JJ. Porcine reproductive and respiratory syndrome virus in mammary secretions. Proc AASP Ann Meet. Des Moines, Iowa. 1998;405-406.
 |










The AASV website and The Journal of Swine Health and Production are made possible by the generous support of the AASV Industry Support Council, including: .
Copyright (C) 1996-2002 American Association of Swine Veterinarians. Please send your suggestions about this site to Dave Brown, webmaster@aasv.org.
This page last updated April 19, 2012.