Diagnostic notes
Update on porcine epidemic diarrhea
Andreas Pospischil, Dr med vet, DECVP; Angela Stuedli, med vet; Matti Kiupel, Dr med vet, PhD, DACVP
Pospischil A, Stuedli A, Kiupel M, et al. Update on porcine epidemic diarrhea. J Swine Health Prod. 2002;10(2):81-85. Also available as a PDF,
for related articles
Editor's note: This article is the fourth in a series dedicated to foreign animal diseases. Although US swine veterinarians are unlikely to see these diseases in practice, awareness is vital to early detection. -- DH Zeman, Diagnostic Notes Editor
AP, AS: Institute for Veterinary Pathology, Faculty of Veterinary Medicine, University of Zurich, Switzerland; MK: Animal Health Diagnostic Laboratory and Department of Pathobiology and Veterinary Diagnostic Investigations, Michigan State University, East Lansing, MI 48824. Address correspondence to Dr Matti Kiupel, Department of Pathobiology and Veterinary Diagnostic Investigation, College of Veterinary Medicine, Michigan State University, G300 Veterinary Medical Center, East Lansing, MI 48824. Tel: 517-432-2670; Fax: 517-355-2152; E-mail: kiupel@ahdl.msu.edu.
This article has not been peer reviewed
Porcine epidemic diarrhea virus (PEDV), a member of the Coronaviridae, is antigenically distinguishablefrom the other porcine coronaviruses, transmissible gastroenteritis virus (TGEV), and hemagglutinating encephalomyelitisvirus (HEV).1,2,3 Diarrhea caused by PEDV is clinically difficult to distinguish from diarrhea caused by infectionwith TGEV.4,5 Transmissible gastroenteritis(TGE)-like outbreaks of acute diarrhea were first reported in feeder and grower pigs in England in 1971, but TGEV and other known enteropathogenic agents were ruled out as the cause.6 During these outbreaks, suckling pigs (less than 4 to 5 weeks of age) did not become sick. In 1976, other European countries reported similar disease outbreaks in pigs of all ages, including suckling pigs.4,7,8 The disease was named "epidemic viral diarrhea" (EVD) and the designates EVD type 1 (EVD1) and EVD type 2 (EVD2) were used to differentiate between the earlier outbreaks and those affecting pigs of all ages.7,8 In 1978, a coronavirus-like agent was demonstrated as the causative agent of EVD2 and probably the cause of EVD1 outbreaks, and the name "porcine epidemic diarrhea" "(PED)" was proposed.7,8 The reason for the different clinical presentations of EVD1 and EVD2 remains unknown. Since then, the disease has been recognized in a number of European countries,and more recently in China, Korea,and Japan.5,9-13 In recent years, acute outbreaks have become rare in regionswhere the virus is widespread. Presentlyin Europe, diarrhea due to PEDV is most often seen in feeder and grower pigs and in young breeding animals, whereas suckling piglets are rarely affected. In Asia, however, severe epizootics with high mortality still occur in swine of all ages, and these outbreaks cannot be differentiated clinically from acute TGE.5,12,13
Characteristics of PEDV
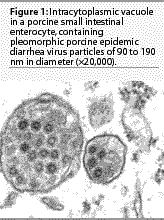 The
morphologic, physicochemical, and biological features of PED virus
particles are characteristic for the coronavirus family.2,8,11
Porcine epidemic diarrhea viral particles are pleomorphic
and range in diameterfrom 90 to 190 nm (Figure 1).2,14-16
They have an electron-dense core with central halo and club-shaped
projections of approximately 20 nm length radiating from the core.2,14-16
The virus is ether- and chloroform-sensitive and does not hemagglutinate
erythrocytes from a wide variety of species.11,17
The
morphologic, physicochemical, and biological features of PED virus
particles are characteristic for the coronavirus family.2,8,11
Porcine epidemic diarrhea viral particles are pleomorphic
and range in diameterfrom 90 to 190 nm (Figure 1).2,14-16
They have an electron-dense core with central halo and club-shaped
projections of approximately 20 nm length radiating from the core.2,14-16
The virus is ether- and chloroform-sensitive and does not hemagglutinate
erythrocytes from a wide variety of species.11,17
Attempts to grow PEDV in cell culture proved difficult, and it took more than 10 years for the virus to be propagated in Vero cells.17,18 Viral growth depends on the presence of trypsin in the cell culture medium. Vero cell-adapted PEDV has been successfully propagated in other cell types.12,19 Only one serotype of PEDV has been identified. Isolates from Europe, Korea,and China are serologically identical to the prototype CV777 strain.4,12,17
The sequence of the entire genome of strain CV777 has only
recently been determined and was found to be 28,033 nucleotides
(nt) in length (excluding the poly
A-tail).20,21Analysis of the nucleotide sequencesrevealed
a small open reading frame (ORF) located near the 5' end (nt 99
to 137), and two large, slightly overlapping ORFs, ORF1a (nt 297
to 12650) and ORF1b (nt 12605 to 20641).20,22,23 The
ORF1a and ORF1b sequences overlap at a potential ribosomal frame
shift site. The amino acid sequence analysis suggests the presence
of several functional motifs within the putative ORF1 protein.
There are majorgenomicdifferences between cell culture-adapted
(ca-PEDV) and wild type virus(wt-PEDV).20-23
Comparative amino acid sequence alignments revealed that PEDV is most closely related to human coronavirus (HCoV) 229E and transmissible gastroenteritis virus (TGEV) and is more distantly related to murine hepatitis virus and infectious bronchitis virus.20,22,24 Antigenic determinants common to PEDV and feline infectious peritonitis virus are located on the nucleocapsid protein.25 Identification of the membrane glycoprotein supports classification as a group I coronavirus.2,11,24,26
Geographic distribution
Porcine epidemic diarrhea was first reported in the UK in 1971, and the virus was identified during episodes of epizootic diarrhea in pigs in Belgium and in the UK.7,8 The disease has subsequently been reported in Canada, Hungary, Germany, China, Korea, and Japan.5,11-13,16 However, outbreaks of severe PED are now rare in Europe. Very likely, this is due to the enzootic character of the virus and the presence of lactogenic immunity, which protects suckling pigs. More recently, severeoutbreaks of PED with high mortality have been reported in Spain, Japan, and Korea.12,27,28 The Asian epizootics are so severe that they cannot be differentiated clinically from acute TGEV outbreaks, and they incur heavy economic losses. Until now, PEDV has not been detected in the United States or South America. Seroprevalence of PEDV has been confirmed in most major swine-producing countries worldwide, but no recent serologicsurveys or diagnostic studies have been published in Europe or North America.10,29
Transmission
Modes of transmission of PEDV are similar to those of TGEV, but PEDV tends to persistmore easily on infected premises. Fecal-oral transmission is probably the main or only route of infection.30,31 Most commonly, the introduction of infected pigs into susceptible farms causes outbreaks of PED within 4 to 5 days.4 Virus may also be introduced through contaminated equipment and other fomites or personnel. After a disease outbreak, PEDV may disappear, or it may become enzootic on farms where there are sufficient litters of pigs to allow the virus to be maintained through infection of consecutive litters that have lost their lactogenic immunity at weaning.
Zoonotic potential and public health implications
Infection of human beings and other species with PEDV has not been reported.
Clinical presentation
The severity of clinical disease caused by PEDV is highly variable, depending largely on the immunological or enzootic status of the pig farm. Nevertheless, the main and often only clinical sign is watery diarrhea.4,7-9,16,30-32 Diarrheic feces are watery and flocculent, and have a characteristic fetid smell owing to steatorrhea.9,16,30-32 Affected animals commonly vomit. In susceptiblebreeding herds, pigs of all ages may be affected, with morbidity approaching 100%. Watery diarrhea, and dehydration and metabolic acidosis causing mortality of 50 to 80%, are the principal features of PED in suckling pigs, whereas infections of feeder and grower pigs are characterized by diarrhea, anorexia, depression, and a high morbidity but low mortality (1 to 3%).9,11,16,30-32 In enzootic situations, PEDV may cause persistent diarrhea in recently weaned pigs. Clinical presentation may be very similar to TGE, but there is often a slower spread of disease and lower mortality in baby piglets, and more severe disease than with TGEV if infection occurs toward the end of the growing period. Mortality in grower pigs may reach 1 to 3%, and pigs often appear to have abdominal pain and may die acutely during the early stages of diarrhea or even prior to its onset.4,8,9,11,16,30-32 Recovery occurs after approximately 7 to 10 days.
Pathogenesis
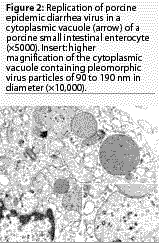 Experimental
oral inoculation of caesarian-derived, colostrum-deprived pigs
with the CV777 isolate of PEDV causes diarrhea 36 hours post inoculation
(PI). Like TGEV, PEDV replicates in the cytoplasm of villous enterocytes
throughout the small intestine (Figure 2), causing epithelial
cell degeneration with subsequent villus shortening.14,16
However, the effects of TGE are more intenseand dramatic.11
Enterocytes along the entire length of villi are susceptible to
infection with PEDV, and infected enterocytes have been observed
as early as 12 to 18 hours PI, with a maximum reached between
24 and 36 hours PI. The clinical course in gnotobiotic pigs experimentallyinfected
with ca-PEDV was much milder than that in pigs infected with wt-PEDV.
The ca-PEDV was notably less virulent, viral replication was slower,
and histopathological changes were less evident.
Experimental
oral inoculation of caesarian-derived, colostrum-deprived pigs
with the CV777 isolate of PEDV causes diarrhea 36 hours post inoculation
(PI). Like TGEV, PEDV replicates in the cytoplasm of villous enterocytes
throughout the small intestine (Figure 2), causing epithelial
cell degeneration with subsequent villus shortening.14,16
However, the effects of TGE are more intenseand dramatic.11
Enterocytes along the entire length of villi are susceptible to
infection with PEDV, and infected enterocytes have been observed
as early as 12 to 18 hours PI, with a maximum reached between
24 and 36 hours PI. The clinical course in gnotobiotic pigs experimentallyinfected
with ca-PEDV was much milder than that in pigs infected with wt-PEDV.
The ca-PEDV was notably less virulent, viral replication was slower,
and histopathological changes were less evident.
Porcine epidemic diarrhea virus has also been detected in epithelial cells of the colonin conventional grower pigs with both experimental and naturally occurring PED.11,16 The significance of colonic infectionand its role in the development of more severe disease in older pigs is unknown. Also, the cause of sudden death accompanied by acute back muscle necrosis that has been described in finishing and adult pigs with PED is unknown. Replication of PEDV in pigs has not been detected in cells outside the intestinal tract.
Pathology
Gross and microscopic lesions are similar to those described for TGE. Stomachs are typically empty due to vomiting, and lacteals are devoid of chyle due to malabsorption. Small intestines are fluid-filled and appear thin-walled due to severe mucosalatrophy (Figures 3 and 4).14,16,28 Intestinalcontents are flocculent. In addition to lesions similar to those of TGE, acute necrosis of back muscle has been reported.
 Microscopically,
marked cytoplasmic vacuolation and exfoliation of enterocytes
with subsequent considerable shortening and fusion of villi is
noted, but is less severe than in TGE (Figures 3, 4, and 5).9,16,32
No microscopic lesions have been observed in the colon. Interestingly,
ultrastructural studies revealed intracytoplasmic viral particlesand
cellular changes in epithelial cells of the small intestine and
the colon.14,16,32 Ultrastructural changes were initially
characterized by loss of cell organelles, microvilli, and the
terminal web, and protrusion of parts of the cytoplasm into the
intestinal lumen.16 Later, cells becameflattened, the
tight junctions were lost, and cells were released into the gut
lumen.
Microscopically,
marked cytoplasmic vacuolation and exfoliation of enterocytes
with subsequent considerable shortening and fusion of villi is
noted, but is less severe than in TGE (Figures 3, 4, and 5).9,16,32
No microscopic lesions have been observed in the colon. Interestingly,
ultrastructural studies revealed intracytoplasmic viral particlesand
cellular changes in epithelial cells of the small intestine and
the colon.14,16,32 Ultrastructural changes were initially
characterized by loss of cell organelles, microvilli, and the
terminal web, and protrusion of parts of the cytoplasm into the
intestinal lumen.16 Later, cells becameflattened, the
tight junctions were lost, and cells were released into the gut
lumen.
Diagnosis
Porcine epidemic diarrhea cannot be diagnosed on the basis of clinical findings alone, and acute outbreaks of PED cannot be clinically differentiated from TGE regardlessof the age of pigs infected. Laboratory methods are necessary for an etiologicdiagnosis. The most commonly used diagnostic methods are the direct immunofluorescence test (IFT) for detection of viral antigen, and enzyme-linked immunosorbant assays (ELISA) to demonstrate PEDV antigen in feces or antibodies in serum.11,27,31,33 The virus can also be detected by immunohistochemistry (IHC),30,34 polymerase chain reaction (PCR),8,24,35-41 indirect fluorescent antibody test (IFA),42 in situ hybridization,43 and electron microscopy (EM).10,16
 Fluorescent
antibody tests and IHC (Figure 6) on sections of small intestine
from pigs with acute diarrhea are the most sensitive, rapid, and
reliable methods. They should be used preferably within 3 days
of onset of diarrhea. Electron microscopy is widely used to detect
viral particles in fecal material of pigs with diarrhea.9,28,30
Identification of coronaviruses in feces may be difficult because
virus particles are not easy to detect if the spikes are lost
or are not clearly visible. Electron microscopy is not a sensitive
technique, and TGEV cannot be differentiated from PEDV. In contrast,the
ELISA has a much higher sensitivity than EM and also allows differentiation
between TGEV and PEDV.27,31,33 Using ELISA tests, PEDV
antigen can be detected in rectal swabs of experimentally inoculated
pigs until 11 days PI.27 The ELISA antigen test, using
monoclonal or polyclonal antibodies, may be used to detectPEDV
in endemically infected herds and cases of persistent diarrhea
in breeding farms where the amount of virus is too small to be
detected by any other methods.27,31,33 More recently,
published reportsshow that reverse transcriptase PCR may be used
in a similar manner to differentiate TGE from PEDV and is highly
Fluorescent
antibody tests and IHC (Figure 6) on sections of small intestine
from pigs with acute diarrhea are the most sensitive, rapid, and
reliable methods. They should be used preferably within 3 days
of onset of diarrhea. Electron microscopy is widely used to detect
viral particles in fecal material of pigs with diarrhea.9,28,30
Identification of coronaviruses in feces may be difficult because
virus particles are not easy to detect if the spikes are lost
or are not clearly visible. Electron microscopy is not a sensitive
technique, and TGEV cannot be differentiated from PEDV. In contrast,the
ELISA has a much higher sensitivity than EM and also allows differentiation
between TGEV and PEDV.27,31,33 Using ELISA tests, PEDV
antigen can be detected in rectal swabs of experimentally inoculated
pigs until 11 days PI.27 The ELISA antigen test, using
monoclonal or polyclonal antibodies, may be used to detectPEDV
in endemically infected herds and cases of persistent diarrhea
in breeding farms where the amount of virus is too small to be
detected by any other methods.27,31,33 More recently,
published reportsshow that reverse transcriptase PCR may be used
in a similar manner to differentiate TGE from PEDV and is highly
sensitive.35-41 Cell culture cannot be used for routine
diagnosis.
Antibodies against PEDV can be detected in sera from swine
with naturally occurring or experimentally induced PED. Anti-
bodies are detectable by blocking ELISA 7 days PI and by IFA testing
between 10 and 13 days PI.27 Paired serum samples should
be examined, and the convalescent sample should be collected 2
to 3 weeks after the onset of diarrhea.17,27,29 Antibodies
generally persist for more than a year in the serumof infected
pigs.
Treatment and prevention
Specific therapeutic recommendations for PED are not available. Symptomatic treatment of diarrhea is recommended, including free access to water to diminish dehydration, and withholding of feed, particularly in growing swine.
Sanitary measures should be taken to preventintroduction of PEDV to the farm. Introduction of persistently infected pigs poses the highest risk, and disease can also be spread by human traffic between affected units. After diagnosis of PED, becauseof the slow spread of disease, the primaryconcern should be initiation of preventive measures to temporarily prevent virus entrance into farrowing units. Artificial exposure of pregnant sows to fecesfrom PEDV-infected pigs stimulates lactogenic immunity and helps to postpone infection of these sows' piglets until they are older, resulting in fewer deaths.11 If persistence of the virus is diagnosed in consecutive litters of weaned piglets after an outbreak has occurred, virus elimination can be attempted by removing pigs immediatelyafter weaning to another site for at least 4 weeks.
Recently, the immunoprophylactic effects of chicken egg yolk immunoglobulin (IgY) against PEDV were investigated in neonatal pigs.42 Administration of IgY was associated with reduced mortality and increased survival rate in piglets after challenge exposure to PEDV. This suggests that IgY against PEDV might be an alternative prophylactic measure similar to stimulated lactogenic immunity.
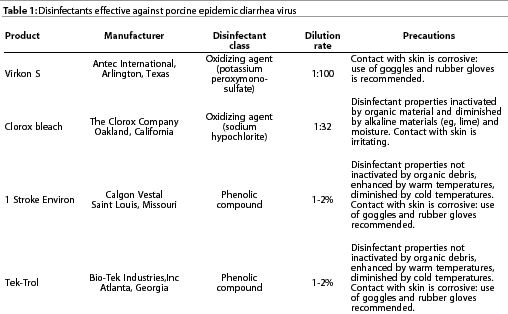
Porcine epidemic diarrhea virus is stable between pH 5.0 and 9.0 at 4°C and between pH 6.5 and 7.5 at 37°C.11 Culture-adapted PEDV loses infectivity when heated to 60°C for 30 minutes, but is moderately stable at 50°C.
Porcine epidemic diarrhea virus is inactivated by most virucidal
disinfectants, including cresol, sodium hydroxide (2%), formalin
(1%), sodium carbonate (4% anhydrousor 10% crystalline, with 0.1%
detergent), ionic and non-ionic detergents, strong iodophors (1%)
in phosphoric acid, and lipid solvents such as chloroform. Examplesof
disinfectants effective against PEDV are shown in Table 1.
References - refereed
1. Cavanagh, D. Classification and nomenclature of virus. Fifth report of the International Committee on Taxonomy of Viruses. In: Francki RIB, Fauquet CM, Knudson DL, Brown F, eds. 1991, Arch Virol. 1991;Suppl 2.
2. Pensaert MB, Debouck P, Reynolds DJ. An immunoelectron microscopic and immuno-fluorescent study on the antigenic relationship between the coronavirus-like agent, CV 777, and several coronaviruses. Arch Virol. 1981;68:45-52.
3. Pensaert MB. Porcine epidemic diarrhea virus. In: Horzinek MC, ed. Virus Infections of Vertebrates. Vol. 2. New York: Elsevier. 1989;167-176.
4. Hess RG, Bollwahn W, Pospischil A, Heinritzi K, Bachmann PA. Current aspects in the etiology of viral diarrheas of swine: occurrence of infections with the epizootic viral diarrhea (EVD) virus. Berl Muench Tieraerztl Wochenschr. 1980;93:445-449.
5. Takahashi K, Okada K, Oshima K. An outbreak of swine diarrhea of a new type associated with coronavirus-like particles in Japan. Japan J Vet Scien. 1983;45:829-832.
7. Chasey D, Cartwright SF. Virus-like particles associated with porcine epidemic diarrhoea. Res Vet Sci. 1978; 25:255-256.
8. Pensaert MB, Debouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978;58:243-247.
9. Debouck P, Pensaert M, Coussement W. The pathogenesis of an enteric infection in pigs, experimentally induced by the coronavirus-like agent, CV 777. Vet Microbiol. 1981;6:157-165.
11. Pensaert MB. Porcine epidemic diarrhea. In: Straw, BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press. 1999:179-185.
12. Kweon CH, Kwon BJ, Jung TS, Kee YJ, Hur DH, Hwang EK, Rhee JC, An SH. Isolation of porcine epidemic diarrhea virus (PEDV) in Korea. Korean J Vet Res. 1993;33:249-254.
13. Qinghua C. Investigation on epidemic diarrhea in pigs in Qinghai region. Qinghai Xumu Shaoyi Zazhi. 1992;22:22-23.
14. Ducatelle R, Coussement W, Debouck P, Hoorens J. Pathology of experimental CV777 coronavirus enteritis in piglets. II. Electron microscopic study. Vet Pathol. 1982;19:57-66.
15. Ducatelle R, Coussement W, Pensaert MB, Debouck P, Hoorens J. In vivo morphogenesis of a new porcine enteric coronavirus, CV 777. Arch Virol. 1981; 68:35-44.
16. Pospischil A, Hess RG, Bachmann PA. Light microscopy and ultrahistology of intestinal changes in pigs infected with epizootic virus diarrhoea (EVD): comparison with transmissible gastroenteritis (TGE) virus and porcine rotavirus infections. Zentralbl Veterinaermed [B]. 1981;28:564-577.
17. Witte KH, Prager D, Ernst H, Neuhoff H. Die epizootische Virusdiarrhoea (EVD). Tieraerztl Umschau. 1981;36:235-250.
18. Hofmann M, Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J Clin Microbiol. 1988;26:2235-2239.
19. Shibata I, Tsuda T, Mori M, Ono M, Sueyoshi M, Uruno K. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet Microbiol. 2000;72:173-182.
20. Duarte M, Tobler K, Bridgen A, Rasschaert D, Ackermann M, Laude H. Sequence analysis of the porcine epidemic diarrhea virus genome between the nucleocapsid and spike protein genes reveals a polymorphic ORF. Virology. 1994;198:466-476.
21. Kocherhans R, Bridgen A, Ackermann M, Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001;23:137-144.
22. Bridgen A, Kocherhans R, Tobler K, Carvajal A, Ackermann M. Further analysis of the genome of porcine epidemic diarrhoea virus. Adv Exp Med Biol. 1998;440:781-786.
23. Duarte M, Laude H. Sequence of the spike protein of the porcine epidemic diarrhoea virus. J Gen Virol. 1994;75:1195-1200.
24. Tobler K, Ackermann M. PEDV leader sequence and junction sites. In: Talbot PJ, Levy GA, eds. Corona and Related Viruses. New York: Plenum Press, 1994.
25. Zhou YL, Ederveen J, Egberink H, Pensaert M, Horzinek MC. Porcine epidemic diarrhea virus (CV 777) and feline infectious peritonitis virus (FIPV) are antigenically related. Arch Virol. 1988;102:63-71.
26. Ducatelle R, Coussement W, Charlier G, Debouck P, Hoorens J. Three-dimensional sequential study of the intestinal surface in experimental porcine CV 777 coronavirus enteritis. Zentralbl Veterinaermed [B]. 1981;28:483-493.
27. Carvajal A, Lanza I, Diego R, Rubio P, Carmenes P. Evaluation of a blocking ELISA using monoclonal antibodies for the detection of porcine epidemic diarrhea virus and its antibodies. J Vet Diagn Invest. 1995;7:60-64.
28. Sueyoshi M, Tsuda T, Yamazaki K, Yoshida K, Nakazawa M, Sato K, Minami T, Iwashita K, Watanabe M, Suzuki Y. An immunohistochemical investigation of porcine epidemic diarrhoea. J Comp Pathol. 1995;113:59-67.
29. Hofmann M, Wyler R. Serologic study of the occurrence of epizootic viral diarrhea in swine in Switzerland. Schweiz Arch Tierheilkd. 1987;129:437-442.
31. Bernasconi C. Epizootische Virusdiarrhoe Virus: Untersuchungen zur Virulenz eines an Zellkultur adaptierten Stammes und Vergleich zweier Nachweismethoden (Immunhistochemie und ELISA). Zurich: Institute of Veterinary Pathology, University of Zurich. 1996:58.
32. Coussement W, Ducatelle R, Debouck P, Hoorens J. Pathology of experimental CV777 coronavirus enteritis in piglets. I. Histological and histochemical study. Vet Pathol. 1982;19:46-56.
33. Knuchel M, Ackermann M, Muller HK, Kihm U. An ELISA for detection of antibodies against porcine epidemic diarrhoea virus (PEDV) based on the specific solubility of the viral surface glycoprotein. Vet Microbiol. 1992;32:117-322.
34. Lee HM, Lee BJ, Tae JH, Kweon CH, Lee YS, Park JH. Detection of porcine epidemic diarrhea virus by immunohistochemistry with recombinant antibody produced in phages. J Vet Med Sci. 2000;62:333-337.
35. Guscetti F, Bernasconi C, Tobler K, Van Reeth K, Pospischil A, Ackermann M. Immunohistochemical detection of porcine epidemic diarrhea virus compared to other methods. Clin Diagn Lab Immunol. 1998;5:412-414.
36. Kim SY, Song DS, Park BK. Differential detection of transmissible gastroenteritis virus and porcine epidemic diarrhea virus by duplex RT-PCR. J Vet Diagn Invest. 2001;13:516-520.
37. Kim O, Choi C, Kim B, Chae C. Detection and differentiation of porcine epidemic diarrhoea virus and transmissible gastroenteritis virus in clinical samples by multiplex RT-PCR. Vet Rec. 2000;146:637-640.
38. Kweon CH, Lee JG, Han MG, Kang YB. Rapid diagnosis of porcine epidemic diarrhea virus infection by polymerase chain reaction. J Vet Med Sci. 1997;59:231-232.
39. Tobler K, Kocherhans R, Ackermann M. Detection of porcine epidemic diarrhea coronavirus by RT-PCR. In: Becker Y, Darai G, eds. PCR: Protocols for Diagnosis of Human and Animal Virus Diseases. Berlin: Springer-Verlag. 1995;483-490.
40. Ishikawa K, Sekiguchi H, Ogino T, Suzuki S. Direct and rapid detection of porcine epidemic diarrhea virus by RT-PCR. J Virol Methods. 1997; 69:191-195.
41. Kubota S, Sasaki O, Amimoto K, Okada N, Kitazima T, Yasuhara H. Detection of porcine epidemic diarrhea virus using polymerase chain reaction and comparison of the nucleocapsid protein genes among strains of the virus. J Vet Med Sci. 1999;61: 827-830.
42. Kweon CH, Kwon BJ, Woo SR, Kim JM, Woo GH, Son DH, Hur W, Lee YS. Immunopro-phylactic effect of chicken egg yolk immunoglobulin (Ig Y) against porcine epidemic diarrhea virus (PEDV) in piglets. J Vet Med Sci. 2000;62:961-964.
43. Kim O, Chae C. In situ hybridization for the detection and localization of porcine epidemic diarrhea virus in the intestinal tissues from naturally infected piglets. Vet Pathol. 2000;37:62-67.
References - non refereed
6. Oldham J. Letter to the editor. Pig Farming. 1972; Oct suppl:72-73.
10. Debouck P, Callebaut P, Pensaert M. Prevalence of the porcine epidemic diarrhea (PED) virus in the pig population of different countries. Proc IPVS. Mexico. 1982.
30. Bernasconi C, Guscetti F, Uttiger A, Van Reeth K, Ackermann M, Pospischil A. Experimental infection of gnotobiotic piglets with a cell culture adapted porcine epidemic diarrhea virus: clinical, histopathological and immunohistological findings. Proc 3rd Cong ESVV. Interlaken, Switzerland. 1995; 542-546.


