Original research
Peer reviewed
Effectiveness of a single intramuscular dose of ceftiofur hydrochloride for the treatment of naturally occurring bacterial swine respiratory disease
David M. Meeuwse, BS; Fabian M. Kausche, DMV; John W. Hallberg, DVM, PhD; W. Lawrence Bryson, PhD; Kenneth J. Dame, BS
Pharmacia Animal Health, 7000 Portage Road, Kalamazoo, MI 49009 Corresponding author: David M. Meeuwse, Pharmacia Animal Health, 7000 Portage Road, Kalamazoo, MI 49001; Tel: 616-833-2639; Fax: 616-833-3295; E-mail: David.M.Meeuwse@Pharmacia.com.
Meeuwse DM, Kausche FM, Hallberg JW, et al. Effectiveness of a single intramuscular dose of ceftiofur hydrochloride for the treatment of naturally occurring bacterial swine respiratory disease. J Swine Health Prod. 2002;10(3):113-117. Also available as a PDF.
Summary
Objective: To evaluate the effectiveness of ceftiofur hydrochloride (HCl) administered once at 5.0 mg ceftiofur equivalents (CE) per kg bodyweight (BW) for treatment of naturally occurring bacterial swine respiratory disease (SRD).
Methods: Pigs (3.6 to 24.5 kg) in eight commercial herds were enrolled in the study. Pigs that exhibited clinical signs of SRD were randomly assigned to receive one intramuscular (IM) injection of either ceftiofur HCl at 5.0 mg CE per kg BW (141 pigs) or placebo (137 pigs) on Day 0. Necropsies were performed and lung lesions scored by a veterinarian on pigs that died and on all surviving pigs on Day 14. Microbiological identification of bacterial organisms was performed on the lungs of all pigs.
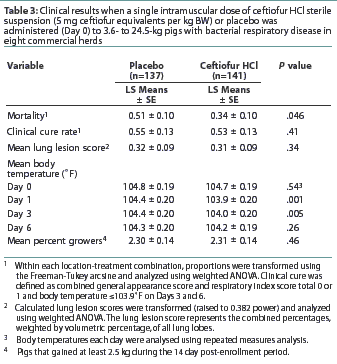
Results: Mortality rate was lower (P<.05) for the ceftiofur-treated pigs (2.1%) than for the placebo-treated pigs (7.3%). Rate of clinical cure (defined prior to the study), lung lesion scores, and percentage of pigs that gained at least 2.5 kg in the 14 days after enrollment did not differ for ceftiofur-treated and placebo-treated pigs. Body temperatures for ceftiofur-treated pigs were lower than for placebo-treated pigs on Days 1 and 3.
Implications: A single IM injection of ceftiofur HCl at 5.0 mg CE per kg BW reduced mortality and body temperature (Days 1 and 3 after injection) for pigs with SRD associated with Actinobacillus pleuropneumoniae, Haemophilus parasuis, Pasteurella multocida, and Streptococcus suis. To effectively treat pigs for SRD, the standard, 3-day, full course therapy of ceftiofur HCl is recommended.
Keywords : swine, respiratory
disease, ceftiofur hydrochloride, mortality.
: swine, respiratory
disease, ceftiofur hydrochloride, mortality.
Received: July 12, 2001
Accepted: November 22, 2001
Swine respiratory disease (SRD) is a result of many factors, and the bacte-rial pathogens associated with it are varied. Bacteria such as Actinobacillus pleuropneumoniae (which causes fibrinous or necrotizing pneumonia), Haemophilus parasuis (the agent of Glasser's disease), Salmonellaserovar Choleraesuis and Pasteurella multocida (causing complicated catarrhal pneumonia), and at least one organismassociated with secondary infection (Streptococcus suis) are susceptible to ceftiofur.1,2,3 Ceftiofur hydrochloride (Excenel RTU Sterile Suspension; Pharmacia Animal Health, Kalamazoo, Michigan) is approved in many countries for treatment of SRD associated with A pleuropneumoniae, P multocida, Salmonella Choleraesuis and S suis. Each mL of Excenel RTU Sterile Suspension contains 50 mg ceftiofur equivalents (CE; expressed as free acid equivalents to indicate that the HCl salt is not included) as ceftiofur HCl. The current label dose in the United States is 3.0 to 5.0 mg CE per kg BW administered IM at 24-hour intervals for 3 consecutive days.
Field experience suggested that a single dose of ceftiofur HCl at 5.0 mg CE per kg BW is an effective treatment for bacterial SRD when treatment occurs at the first signs of respiratory distress. During the development of ceftiofur HCl, a single dose of 1.0 mg CE per kg BW significantly reduced mortality in 13- to 20-kg pigs challenged with A pleuropneumoniae, but was less effective than doses of 5.0, 10.0, or 25.0 mg CE per kg BW at reducing body temperature, lung lesion scores, and clinical signs of pneumonia, or increasing feed consumption and rate of weight gain.4 A dose-response study determined that when ceftiofur HCl was administered one time, 5.0 mg CE per kg was optimal (compared with doses of 0, 1.0 and 3.0 mg CE per kg) at reducing mortality and lung lesion scores in swine with respiratory disease in an A pleuropneumoniae-challenge model.5
In a pharmacokinetic study, plasma concentrations of ceftiofur and active ceftiofur metabolites were 0.274 +/- 0.055 mg per mL at 96 hours after a single IM injection of ceftiofur HCl (5.0 mg CE per kg BW) in healthy 28- to 77.6-kg pigs.6 Salmon et al1 reported in vitro ceftiofur minimum inhibitory concentrations for 90% of the isolates (MIC90) of <=0.03 mg per mL for A pleuropneumoniae and P multocida and 0.25 mg per mL for S suis. These results are similar to those of a previous study that documented an MIC90 of <=0.125 mg per mL for A pleuropneumoniae and P multocida.2 In addition, Salmon et al3 identified an MIC90 of 0.06 mg per mL for H parasuis. Taken together, these findings suggest that after a single injection of ceftiofur HCl at 5.0 mg CE per kg BW, plasma concentrations of ceftiofur and active ceftiofur metabolites will exceed the MIC90 for A pleuropneumoniae, P multocida, S suis, and H parasuis for 96 hours (4 days). The study described herein was designed to evaluate the effectiveness of a single dose of ceftiofur HCl at 5.0 mg CE per kg BW for the treatment of bacterial SRD in 3.6- to 24.5-kg pigs under field conditions.
Materials and methods
Facilities
This study was conducted in eight commercial herds in the midwestern United States (Iowa, Minnesota, Nebraska, and Indiana). Prior to the study, it was determined that eight locations with at least ten pigs per treatment group within location were expected to provide 80% power to detect a 10% difference in mortality with a 95% confidence level. Facilities included nurseries that housed 50 to 530 pigs per room in pens of 17 to 38 pigs per pen, and one grower facility that housed 400 pigs per room in pens of 20 pigs each. All facilitieshad either wire mesh decking or slatted floors. Ventilation was provided by either sidewall or ceiling ventilators and all units maintained temperature with propane heaters. Pig stocking density at the beginning of the study ranged from approximately 0.2 to 0.5 m2 per pig.
Herd enrollment criteria included a history of bacterial SRD and the ability to enroll at least 23 pigs within 7 days. All eight herds were identified by cooperating investigators as having a history of bacterial SRD, and in each herd, 26 to 43 pigs were enrolled in 2 days. While some sites had a history of porcine reproductive and respiratory syndrome or swine influenza, no sites had a history of pseudorabies. We did not discriminate against sites with a history of viral respiratory disease nor did we conduct serology to determine what viral respiratory pathogens may have been present in each herd, but rather enrolled pigs that showed clinical signs of SRD and used results of microbiological tests on lung tissue to confirm bacterial infection.
Study animals
Both male and female crossbred weaned pigs (3.6 to 24.5 kg) were enrolled after a 14-day period when no parenteral, in-feed, or in-water medication was administered. During this prohibition period (which in some cases occurred directly after weaning) and during the course of the study, samples of feed were taken from each batch of feed used, and antibiotic assays were performed at a commercial laboratory (Barrow-Agee Laboratories, Memphis, Tennessee).
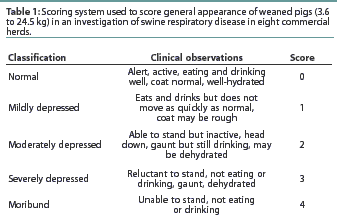
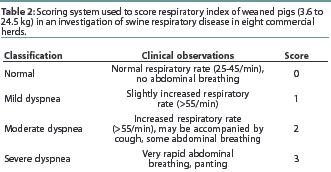
In each herd, enrollment began when a minimum of 23 pigs exhibited clinical signs of SRD; treatment was administered at enrollment. Pigs were enrolled by the herd veterinarian if they had a body temperature >=104.0°F, and combined general appearance score (Table 1) and respiratory score (Table 2) totaling 2 or greater.
Sentinel pigs
At each site, three sentinel pigs (non-treated pigs that met entrance criteria) were sacrificed before treatment. The lungs of sentinel pigs were submitted for microbiological diagnostic evaluation (Veterinary Diagnostic Laboratory, Iowa State University, Ames, Iowa) to confirm the presence of bacterial SRD in the herd at the time of treatment.
Study design
The study was conducted as a randomized complete block design, with two pigs per block and between 12 and 20 complete blocks enrolled at each site. All study personnel except the treatment administrator were blinded to treatment throughout the trial. The treatment administrator recorded no observations throughout the study except those associated with treatment of the pigs.
Treatment
Eligible pigs were randomly assigned to receive one IM injection
of either ceftiofur HCl (5.0 mg CE per kg BW) or sterile
water of the same volume (0.1 mL per kg BW) at enrollment on Day
0. A new plastic syringe and 3.75-cm, 18-gauge needle were used
to administer each post-auricular injection in the neck muscle.
Enrolled pigs were ear-tagged and returned to their home pen after
treatment, ie, ceftiofur-treated pigs, placebo-treated pigs, and
non-study pigs were housed together.
Clinical observations and necropsies
Clinical observations were noted daily for 7 days, and body temperatures were recorded on Days 1, 3, and 6. Necropsies, including gross observations of all body systems, were performed and lung lesions were scored by the herd veterinarian on the three sentinel pigs at each location prior to treatment administration, on all pigs that died during the study, and on surviving pigs that were euthanized at the end of the study, 14 days after enrollment. Microbiological identification of bacterial organisms was performed on the lungs of all enrolled pigs (Veterinary Diagnostic Laboratory, Iowa State University).
Calculations
The three primary variables were mortality, clinical cure rate, and lung lesion scores. Mortality was calculated as the percentage of pigs that died as a result of SRD, or became moribund due to SRD and were euthanized during the 14-day post-treatment period. Only pigs diagnosed with pneumonia or respiratory disease at necropsy were included in the mortality analysis. A pig was considered clinically cured if the combined general appearance score and respiratory index score totaled 0 or 1 and the body temperature was <=103.9°F on Days 3 and 6. The lung lesion score represented the combined percentages, weighted by volumetric percentage, of all lung lobes. Growth, measured as percent "growers," was an ancillary variable which was included to determine whether treated animals continued to grow, and which served as a reflection of general health. Percent growers was defined as the percentage of pigs that survived the entire 14-day observation period and gained >=2.5 kg.
Statistical analysis
Mortality rate, clinical cure rate, and percent growers within each location and treatment group were transformed using the Freeman-Tukey arcsine transformation to satisfy the assumptions of the analysis of variance.7 The transformed values were analyzed using weighted ANOVA where the weights were n + 0.5, and n was the number of animals within the location by treatment group. The lung lesion scores were also transformed prior to analysis (raised to 0.382 power). The model for analysis included the fixed effect of treatment and the random effects of location, location by treatment, and residual. Body temperatures were statistically analyzed using a repeated measures analysis that included the fixed effects of treatment and day and the random effect of location, as well as all interactions of these effects. All statistical analyses were conducted utilizing the PROC MIXED procedure from SAS.8 Eight herds and 278 non-sentinel pigs were included in the statistical analysis.
Results
Feed assay
At four of the eight locations, small quantities of either chlortetracycline (<=9 g per ton) or oxytetracycline (<12 g per ton) or both were present in the feed during either the acclimatization period or the enrollment period.
Mortality
Mortality occurred in five of the eight herds. Mortality rate was 2.1% (3 of 141 pigs) for the ceftiofur-treated pigs and 7.3% (10 of 137 pigs ) for the placebo-treated pigs, and transformed mortality rate was lower (P<.05) for ceftiofur-treated pigs than for placebo-treated pigs (Table 3). All deaths included in the mortality analysis were associated with SRD as confirmed by necropsy. One placebo-treated pig was not included in the mortality analysis. Although this pig had clinical signs consistent with SRD at enrollment and a lung lesion score of 10.25% (representing the combined percentages, weighted by volumetric percentage, of all lung lobes) at necropsy when it died on Day 9, bacterial organisms were not isolated from the lungs, µ-hemolytic streptococci were isolated from brain tissue, and the diagnosis was bacterial meningitis.
Clinical observations and necropsies
Clinical results are shown in Table 3.
The mean clinical cure rates were 7.1% for ceftiofur-treated pigs and 7.3% for placebo-treated pigs. Transformed means did not differ (P>.05).
Mean body temperature of ceftiofur-treated pigs did not differ from that of placebo-treated pigs at enrollment on Day 0 or on Day 6, but was lower for ceftiofur-treated pigs than for placebo-treated pigs on Day 1 and Day 3.
The percentage of ceftiofur-treated pigs that gained at least 2.5 kg in the 14 days after enrollment (mean 81.9%) did not differ from that of placebo-treated pigs (mean 83.5%).
Mean lung lesion incidence (combined percentage, weighted by volumetric percentage, of all lung lobes) was 13.3% for pigs receiving ceftiofur HCl and 11.0%. for pigs receiving placebo, and the transformed means for the two groups did not differ. The lungs from one of the 137 placebo-treated pigs were inadvertently not submitted for microbiological evaluation.
Microbiological identification of pathogens
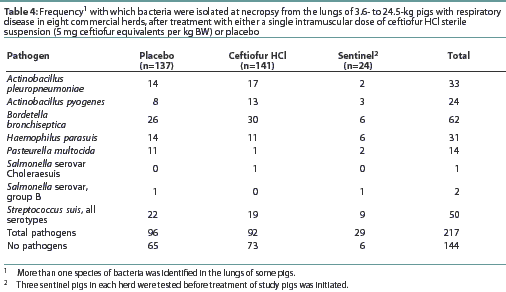
Results of the microbiological evaluations of lungs are presented in Table 4. Actinobacillus pleuropneumoniae, P multocida, H parasuis, and S suis were represented in both the pre-treatment sentinel pigs and in post-treatment pigs. Bordetella bronchi-septica, a pathogen not susceptible to ceftiofur in vitro, was the most frequently identified pathogen in study pigs (Table 4). There was no reduction in the frequency of isolation of these pathogens from the lungs of ceftiofur-treated compared to placebo-treated pigs (Table 4).
Discussion
Four of the bacterial species isolated from the lungs of pigs in this trial (A pleuropneu-moniae, P multocida, S suis, and H parasuis) are known to be susceptible to ceftiofur1-3 and occurred with sufficient frequency across the study herds to confirm that these herds were infected with organisms susceptible to ceftiofur. Salmonella Choleraesuis, which is also susceptible to ceftiofur, was isolated from only one lung, too infrequently to establish clinical effectiveness. While no statistical analyses were conducted on the prevalence of these microorganisms, the fact that there was no reduction in the number of ceftiofur-treated pigs infected with these pathogens is understandable in this study, which allowed study pigs to cohabit with non-study pigs, which could, conceivably, repeatedly infect study pigs.
The most frequently identified pathogen in the lungs of these pigs (B bronchiseptica) is not susceptible to ceftiofur, which may explainwhy there was no improvement in clinical cure rate for ceftiofur-treated pigs.
The amounts of chlortetracycline and oxy-tetracycline present
in the feed during either the acclimatization or enrollment period
were well below the therapeutic levels for either antibiotic (22
mg per kg BW,
400 g per ton in feed). These antibiotics, which were
present in the feed for both experimental groups, are unlikely
to have had any effect on the outcome of the study.
The significant reduction in mortality among ceftiofur-treated pigs in this study establishes a single IM dose of ceftiofur HCl as an alternate treatment regimen (compared to the full-course, 3-day therapy) to reduce mortality caused by SRD. While the reduction in body temperature of approximately 0.5 F° on Day 1 does not appear to be biologically significant, it was statistically significant and certainly indicates a reduction in severity of clinical signs of SRD. However, clinical cure rates, lung lesion scores, and percentage of pigs gaining at least 2.5 kg were not statistically different for treated and control pigs, and no claim for the treatment of swine SRD with a single injection of ceftiofur HCl at 5.0 mg CE per kg is implied. Rather, the full-course, 3-day therapy should be utilized.
An alternate study design, including a group treated with the label dosing regimen of 3.0 to 5.0 mg CE per kg BW, would allow direct comparison of the full- course therapy with the single-dose administration in the presence of a placebo-treated control group. In this study, conducted under field conditions and therefore likely to be predictive of field results, mortality in pigs with SRD was reduced when they were treated with a single IM injection of ceftiofur HCl at 5.0 mg CE per kg BW, but SRD was not clinically cured.
Implications
- A single IM injection of ceftiofur HCl at 5.0 mg CE per kg BW significantly reduced mortality and body temperature on post-treatment days 1 and 3 in 3.6- to 24.5-kg pigs with swine respiratory disease (SRD) associated with A pleuropneumoniae, H parasuis, P multocida, and S suis.
- To effectively treat pigs for SRD associated with A pleuropneumoniae,
- H parasuis, P multocida, and S suis, the 3-day label dosage of ceftiofur HCl is recommended.
Acknowledgments
We thank the veterinarians who conducted the study: Dr Alan Beyer, West Branch, Iowa; Dr Darren Bryant and Dr Ron Harrison, Brownstown, Indiana; Dr Jeff Harker, Frankfort, Indiana; Dr John Johnson, Rock Valley, Iowa; Dr Kenneth Lorenzen and Dr John Waddell, Sutton, Nebraska; Dr Steve Menke, Ottumwa, Iowa; Dr Paul Yeske and Dr Martin Mohr, St Peter, Minnesota. We also thank the swine owners and their employees who participated in the study.
References - refereed
1. Salmon SA, Watts JL, Case CA, Hoffman LJ, Wegener HC, Yancy RJ Jr. Comparison of MICs of ceftiofur and other antimicrobial agents against bacterial pathogens of swine from the United States, Canada and Denmark. J Clin Microbiol. 1995;33:2435-2444.
2. Raemdonk DL, Tanner AC, Tolling ST, Michener SL. Antimicrobial susceptibility of Actinobacillus pleuropneumoniae, Pasteurella multocida and Salmonella choleraesuis isolated from pigs. Vet Rec. 1994;134:5-7.
6. Brown SA, Hanson BJ, Mignot A, Millerioux L, Hamlow PJ, Hubbard VL, Callahan JK, Kausche FM. Comparison of plasma pharmacokinetics and bioavailability of ceftiofur sodium and ceftiofur hydrochloride in pigs after a single intramuscular injection. J Vet Pharmacol Therap. 1999;22:35-40.
7. Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607-611.
References - non refereed
3. Salmon SA, Libal MC, Klinefelter T, Bosch GJ, Bradford JR, Watts JL, Hoffman LJ. Minimum inhibitory concentration determination for ceftiofur, spectinomycin and lincomycin/spectinomycin 1:2 against Actinobacillus suis and Haemophilus parasuis. Allen D. Lehman Swine Conf Res Abstr. 1998;25(suppl):31.
4. Evans RA, Uhlenhopp EK, Hoffman LJ, Yancy RJ Jr, Paulissen JB. Ceftiofur hydrochloride, a new broad spectrum cephalosporin: effectiveness against induced Haemophilus pleuropneumoniae of growing swine [abstract]. Proc IPVS. Rio de Janeiro, Brazil. August 1988.
5. Uhlenhopp EK, Hoffman LK, Niyo Y, Evans RA. Clinical trials evaluating ceftiofur hydrochloride against induced Haemophilus pleuropneumoniae. Proc AASP. St Louis, Missouri. March 1988.
8. SAS Institute Inc. SAS/STAT User's Guide, Version 8. Cary, North Carolina: SAS Institute; 1999.
9. Arrioja-Dechert A, ed. Compendium of Veterinary Products. 6th ed. Port Huron, Michigan: North American Compendiums Inc; 2001:1110, 2149.
