Original research
Peer reviewed
Experimental injection of gilts with porcine reproductive and respiratory syndrome virus (PRRSV) during acclimatization
Laura Batista, DVM; Carlos Pijoan, DVM, PhD; Montserrat Torremorell, DVM, PhD
LB, CP: Clinical and Population Science, University of Minnesota, College of Veterinary Medicine, St Paul, MN 55108. MT: Pig Improvement Company, Franklin, KY 43124.
Batista L, Pijoan C, Torremorell M. Experimental injection of gilts with porcine reproductive and respiratory syndrome virus (PRRSV) during acclimatization. J Swine Health Prod. 2002;10(4):147-150. Also available as a PDF.
Summary
Objective: To evaluate the serological patterns of seroconversion in seronegative and seropositive replacement gilts after inoculation with the homologous strain of porcine reproductive and respiratory syndrome virus (PRRSV) as a method of acclimatiza-tion.
Methods: In five herds, groups of replacement gilts that differed in PRRSV serological and exposure status were monitored by PRRS ELISA during the isolation-acclimatization period. Prior to arrival and isolation of the replacement animals, a potential source of herd-specific PRRSV was located. In each nursery, blood was collected 1 week after seroconversion from pigs that were presumed to be PRRSV-viremic. Serum from these animals was used as the inoculum for the replacement gilts. Five days post arrival (Day 0), gilts in Category A (seronegative gilts) and Category B (sero-positive gilts) received an intramuscular injection of 2 mL of the serum preparation. Animals in Category C (seropositive gilts) were not exposed to PRRSV during acclimatization.
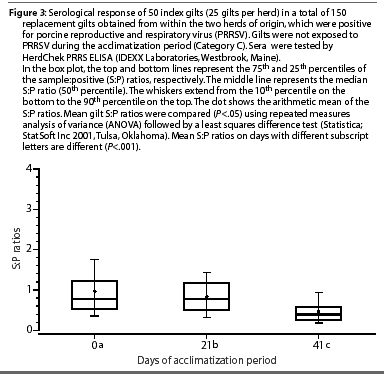
Results: All Category A gilts seroconverted by 21 days post-inoculation, and ELISA sample:positive ratios decreased approximately 7 weeks after inoculation. Category B and C gilts had mixed responses after 21 days. Sample:positive ratios increased in the seropositive, inoculated gilts (Category B) and decreased in the seropositive, non-inoculated group (Category C).
Implications: Inoculation of PRRSV-negative replacement gilts with serum from nursery pigs presumed to be PRRSV-viremic resulted in seroconversion of all 50 gilts tested.
Keywords: swine, porcine respiratory and reproductive syndrome virus, acclimatization
swine, porcine respiratory and reproductive syndrome virus, acclimatization
Received: August, 14, 2001
Accepted: November 9, 2001
Porcine reproductive and respiratory syndrome (PRRS) constitutes one of the most important disease problems that the swine industry faces today.1 Estimated losses in infected herds in the United States are US$252 per sow per year due to increased mortality, reduced growth rates, and augmented medication and vaccination costs.1
Porcine reproductive and respiratory syndrome is caused by an RNA virus (PRRSV) belonging to the Family Arteriviridae,2 which also includes lactate dehydrogenase elevating virus of mice, equine arteritis virus, and simian hemorrhagic fever virus. Porcine reproductive and respiratory syndrome is characterized by reproductive failure, death in young pigs, and mild respiratory disease in swine of all ages. The main risk factors for introduction of PRRSV into naive herds or reintroduction into previously infected herds are the purchase of semen, breeding stock, or both. Recently, issues related to biosecurity (eg, transportation) have been mentioned as important factors in the spread of the disease from positive to naive herds.3 Naive gilts introduced into a seropositive herd are susceptible to PRRSV, but seronegative and even seropositive sows are also susceptible if exposed to a heterologous strain of virus.4 Gilt acclimatization, the single most important and effective management scheme to control PRRSV infection,5,6 is necessary also as a preamble to eradication, to prevent recirculation of the virus in the sow herd. Several investigators report that up to 15% of the adult population may remain seronegative 2 to 3 months after natural herd exposure to PRRSV.7,8 Persistent infection (defined as "the continued presence of a pathogen in a host beyond the acute symptomatic phase of infection")9,10 has been detected up to 157 days after experimental infection in weaned pigs.11 Persistence in adult sows may be shorter (up to 86 days).12 If such animals shed the virus, naive animals may become infected, resulting in irregular periods of virus circulation and clinical PRRS in the herd.12
Gilt acclimatization,13 partial depopulation,1 herd stabilization,14 and test and removal15 are among the approaches described to control PRRSV infection and eradicate PRRS from pig herds. Increasing evidence indicates that PRRSV strains differ in virulence and are biologically, antigenically, and genetically heterogeneous.16 Therefore, it appears that the vaccines currently available commercially in the United States, that contain a single strain of PRRSV, may not be effective in protecting against infections with genetically different strains of PRRSV.16 In addition, many countries do not allow the use of live PRRS vaccines. Consequently, procedures that expose gilts to the homologous herd strain have represented a successful approach being implemented in many countries. Shibata et al17 showed that after exposure to a specific strain of PRRSV, pigs subsequently challenged with that strain did not develop clinical signs, and virus proliferation was reduced both in titer and in length of infection. Lager et al18 reported that intrauterine inoculation of gilts at breeding protected them against infection with the same PRRSV isolate administered by intranasal inoculation in late gestation.
The objective of this study was to evaluate the serological patterns of seroconversion (PRRS ELISA) in seronegative and sero-positive replacement gilts after inoculation with the homologous strain of PRRSV in nursery pig serum as a method of acclimatization.
Materials and methods
Animals and housing
Replacement gilts in five herds were either obtained from within the herds or purchased from outside sources. In each herd, the 150 gilts used in the study were housed in an isolation-acclimatization building belonging to the recipient farm, but separate from other animals in the herd. Similar biosecurity measures were used in the isolation units of all farms. Distance to the recipient or any swine farm ranged from 3 to 30 km. All isolation facilities were naturally ventilated, bird-proofed, and managed as all in-all out buildings.
Study design
Gilt categories. Gilts were assigned to one of three categories, depending on PRRSV serological status (positive or negative) and inoculation with PRRSV, and 50 index animals were randomly selected in each category for serological testing. On each farm, these index animals remained housed with the rest of the group of replacement gilts.
Category A (PRRSV-seronegative) consisted of 50 gilts randomly selected from the isolation-acclimatization building of a PRRSV-positive herd that purchased gilts from a PRRSV-negative source, then exposed them during acclimatization to the presumed source of the herd's homologous strain of PRRSV.
Category B (PRRSV-seropositive), consisted of a total of 50 randomly selected gilts from the isolation-acclimatization buildings of two PRRSV-positive herds (25 gilts from each herd) which obtained PRRSV-seropositive gilts from their own internal multiplier or finishing barns and then exposed them during acclimatization to the presumed source of each herd's homologous strain of PRRSV.
Category C (PRRSV-seropositive) consisted of a total of 50 randomly selected gilts from the isolation-acclimatization buildings of two PRRSV-positive herds (25 gilts from each herd), which obtained them from their own internal multiplier or finishing barns but did not deliberately expose them to PRRSV.
Inoculation with nursery pig serum. Gilts were received directly into each farm's acclimatization facility and individually identified upon arrival using numbered ear tags. Five days post arrival (Day 0), all gilts in Categories A and B received an intramuscular injection of serum collected from nursery pigs in their respective herds (PRRSV inoculum). Animals in Category C were neither exposed to the PRRSV inoculum nor sham-inoculated.
Blood collection. Blood samples were collected from the 50 index gilts in each category (N=150) for serological testing on Days 0, 21, and 42 post exposure. Samples were also collected from animals in Category B on Days 63 and 84. Blood was centrifuged and serum was stored at -20°C until tested.
Inoculum preparation
For each herd, prior to the arrival and isolation of the replacement animals at the isolation-acclimatization building, a potential source of the herd-specific strain of PRRSV was located. In each herd, blood samples were collected from nursery animals for serological profiling for PRRSV antibodies. One week after seroconversion, seropositive pigs from each nursery were selected as a potential source of field virus.19 Blood samples were drawn from these piglets from the anterior vena cava by venipuncture and stored on ice during collection and transportation to the laboratory. Serum was harvested by centrifugation for 10 minutes at 850g; gentamicin was added to the serum at 5 mg per L to prevent growth of bacterial contaminants. Serum preparations were stored at -20°C. On the basis of a study by Cuartero et al,19 the nursery pig sera used to inoculate the study gilts were estimated to have PRRSV titers between 102 and 104 median tissue culture infective doses per mL.
Serology
Blood samples collected from nursery pigs and gilts were tested by PRRS ELISA (HerdChek PRRS ELISA; IDEXX Laboratories, Westbrook, Maine). Titers were expressed as sample: positive (S:P) ratios, with values >=0.4 considered positive.20
Statistical analysis
Mean gilt S:P ratios within each gilt category were compared (P<.05) using repeated measures analysis of variance (ANOVA) followed by a least squares difference test (Statistica; StatSoft Inc 2001, Tulsa, Oklahoma).
Results
Gilts in Categories A and B were depressed, anorexic, and feverish (body temperature 40 to 41.5°C) for approximately 48 to 72 hours post inoculation, while the animals in Category C showed no clinical signs. No mortality was observed in any group.
At 21 days post challenge, all of the previously PRRSV-seronegative, inoculated gilts (Category A) had seroconverted, ie, had S:P ratios >=0.4, as shown in Figure 1. Mean S:P ratios were higher on Day 21 than on Day 0 (P<.001). By Day 42, mean S:P ratios had declined and were lower (P<.001) than on Day 21, but remained higher (P<.001) than on Day 0. Category A gilts were moved into the farm's breeding and gestation area 1 week after the Day 42 sample was collected.
Gilts in the seropositive categories had mixed responses at Day 21. Mean S:P ratios of gilts in Category B (PRRSV-seropositive, inoculated) are shown in Figure 2. Mean S:P ratios were higher on Days 21, 42, and 63 compared to Day 0 (P=.006). The mean S:P ratio continued to increase after Day 21 and was higher on Day 42 than on Day 21 (P=.006), but declined by Day 63 (P=.006). All Category B gilts with declining S:P ratios on Day 63 were moved to the corresponding farm's breeding and gestation area 1 week later. On Day 84, the S:P ratios continued to be high in 15 to 20% of the remaining Category B animals (data not shown), which were culled from the herd.
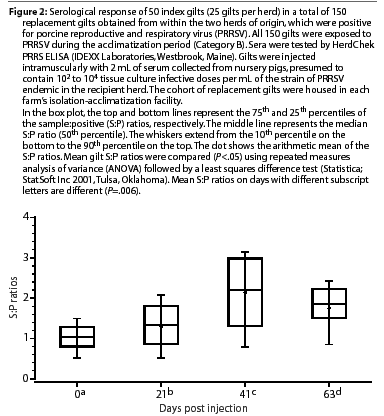
The mean S:P ratios of gilts in Category C (PRRSV-seropositive, non-inoculated) are shown in Figure 3. Mean S:P ratios were lower on Day 21 and Day 42 compared to Day 0 (P<.001).
Discussion
The objective of this study was to evaluate PRRSV ELISA S:P ratios of seronegative and seropositive replacement gilts inoculated with nursery pig serum (presumed source of PRRSV) as a method of acclimatization to PRRSV. All of the previously seronegative index gilts (Category A) were seropositive at 21 days post challenge, and as S:P ratios were declining by Day 42, it was concluded that most of the gilts were no longer viremic and could be moved into the farm's breeding and gestation area. The increase in S:P ratios in Group B gilts on Days 21 and 42 post inoculation was surprising, as a null or negative change in S:P ratios after homologous challenge has been previously reported.21 In view of this unexpected response, additional blood samples were collected from index gilts in Group B at 63 and 84 days post inoculation, and some gilts still had high S:P ratios. It was decided to cull these gilts, as it was possible that they were still viremic, representing a high risk for introduction of PRRSV into the herd.5 These previously seropositive animals might have been safely introduced after a longer period in isolation, ie, 13 weeks.22
It is unclear why gilts in Category B showed a secondary antibody response, when animals challenged with a homologous strain of PRRSV under experimental conditions usually do not.21 It might be argued that inoculation with serum from nursery pigs does not constitute a homologous challenge, as more than one viral strain might be present in the herd.4 The results in this study might have been different because PRRSV titers in weaned pigs tend to be higher than those commonly used in experimental challenges.19 Virus isolation, sequencing, and titration were not performed on the nursery pig sera, so the issues of whether the inoculum for each group of gilts contained more than one strain of PRRSV and what the exact viral titer was in each inoculum remain unresolved.
Inoculation of replacement gilts with PRRSV-infected pig serum was successful in achieving seroconversion in these animals. However, this method of exposure obviously carries some risks, because serum from nursery pigs may contain other pathogens. This is especially important in areas where classical swine fever is still a problem. Also, it is critical that the acclimatization unit be placed in an isolated site, away from the rest of the herd, to
prevent accidental re-infection of the sow herd or introduction of new strains of PRRSV.
Assessment of S:P ratios is not a reliable indicator that either seronegative animals (Category A in this study) or seropositive, non-exposed animals (Category C in this study) are not viremic and do not represent a source of infection to the recipient herd. There is no evidence that S:P ratios correlate well with viremia or virus shedding.23 Finally, it is important that further research using PRRS bioassay be undertaken to determine whether the seropositive, inoculated animals (Category B) that had high S:P ratios throughout the trial period represented a reservoir of PRRSV and a source of infection, since culling these persistently seropositive animals is a detriment to the profitability of the herd.
Implications
- Inoculation of PRRSV-negative replacement gilts with serum collected from nursery pigs presumed to be PRRSV-viremic resulted in seroconversion of all 50 gilts tested.
- This method was successful in exposing replacement gilts to the herd's homologous strain or strains of PRRSV during acclimatization.
References - refereed
1. Dee SA, Joo HS, Polson DD, Marsh WE. Evaluation of the effects of nursery depopulation of the profitability of 34 pig farms. Vet Rec. 1997;140:498-500.
2. Cavanaugh D, Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;142:629-633.
4. Dee S, Torremorell M, Rossow K, Mahlum C, Otake S, Faaberg K. Identification of genetically diverse sequences (ORF 5) of porcine reproductive and respiratory syndrome virus in a swine herd. Can J Vet Res. 2001;65:254-260.
6. Dee S. An overview of production systems designed to prepare naïve replacement gilts for impending PRRSV challenge. A global perspective. J Swine Health Prod. 1997;5:231-239.
8. Wensvoort G, de Klyver EP, Pol JM, Wagenaar F, Moormann RJ, Hulst MM, Bloemraad R, den Besten A, Zetstra T, Terpstra C. Lelystad virus, the cause of porcine epidemic abortion and respiratory syndrome: a review of mystery swine disease research at Lelystad. Vet Microbiol. 1992;33:185-193.
9. Ahmed R, Morrisono L, Knipe D. Persistence of viruses. In: Fields B, Knipe D, Howley P, eds. Field's Virology. 3rd ed. Philadelphia: Lippencott-Raven. 1996;219-249.
10. Ahmed R, Morrison L, Knipe D. Viral persistence. In: Nathanson N, Ahmed R, Gonzalez-Scarano F, Griffin D, Holmes K, Murphy F, Robinson H, eds. Viral Pathology. Philadelphia: Lippincott-Raven. 1997;181-206.
11. Wills RW, Zimmerman JJ, Yoon KJ, Swenson SL, McGinley MJ, Hill HT, Platt KB, Christopher-Hennings J, Nelson EA. Porcine reproductive and respiratory syndrome virus: a persistent infection. Vet Microbiol. 1997;55:231-240.
12. Bierk M, Dee S, Rossow K, Collins J, Otake S, Molitor T. Transmission of PRRS virus from persistently infected sows to contact controls. Can J Vet Res. 2001;65:261-266.
15. Dee SA, Molitor TW, Rossow KD. Epidemiological and diagnostic observations following the elimination of porcine reproductive and respiratory syndrome virus from a breeding herd of pigs by the test and removal protocol. Vet Rec. 2000;146:211-213.
16. Meng XJ. Heterogeneity of porcine reproductive and respiratory syndrome virus: implications for current vaccine efficacy and future vaccine development. Vet Microbiol. 2000;74:309-329.
17. Shibata I, Mori M, Yazawa S. Experimental reinfection with homologous porcine reproductive and respiratory syndrome virus in SPF pigs. J Vet Med Sci. 2000;62:105-108.
18. Lager KM, Mengeling WL, Brockmeier SL. Evaluation of protective immunity in gilts inoculated with the NADC-8 isolate of porcine reproductive and respiratory syndrome virus (PRRSV) and challenge-exposed with an antigenically distinct PRRSV isolate. Am J Vet Res. 1997;60:1022-1027.
20. Meier W, Wheeler J, Husmann R, Osorio F, Zuckermann F. Characteristics of the immune response of pigs to PRRS virus. Vet Res. 2000;31:41.
21. Joo HS, Park BK, Dee SA, Pijoan C. Indirect fluorescent IgM antibody response of pigs infected with porcine reproductive and respiratory syndrome virus. Vet Microbiol. 1997;55:303-307.
22. Batista L, Dee S, Rossow K, Deen J, Pijoan C. Assessing the duration of porcine reproductive and respiratory syndrome virus persistence and shedding in a large population of breeding age female swine. Can J Vet Res. In press.
23. Mengeling WL, Lager KM. A brief review of procedures and potential problems associated with the diagnosis of porcine reproductive and respiratory syndrome virus. Vet Res. 2000;31:61-69.
References - non refereed
13. Dee S. Gilt development and PRRS: A model program for the US swine industry. Compend Cont Educ Pract Vet. 1997;19(9):S228-S232, S237.
14. Dee S. Control and eradication of porcine reproductive and respiratory syndrome. Compend Cont Educ Pract Vet. 2000;22(2):S27-S35.
