Original research |
Peer reviewed |
Using serology in combination with acute phase proteins and cortisol to determine stress and immune function of early-weaned pigs
Gene F. Grellner, DVM, MS; Thomas J. Fangman, DVM, MS, Diplomate ABVP; Jeffery A. Carroll, PhD; Charles E Wiedmeyer, DVM, PhD
GFG: Countryside Veterinary Clinic, Loose Creek, Missouri; TJF, CEW: University of Missouri, Columbia, Missouri; JAC: Animal Physiology Research Unit, ARS-USDA, Columbia, Missouri.
Grellner GF, Fangman TJ, Carroll JA, et al. Using serology in combination with acute phase proteins and cortisol to determine stress and immune function of early-weaned pigs. J Swine Health Prod. 2002;10(5):199-204. Also available as a PDF.
Summary:
Objectives: To evaluate serum concentrations of cortisol and acute phase proteins (APPs), specifically alpha 1-acid glycoprotein (AGP) and haptoglobin (HPT), in the same early-weaned pigs over time and to determine whether changes in these APPs or cortisol are associated with health status and performance in pigs.
Methods: Groups of pigs were evaluated in two management systems, wean-nursery-finish (WNF; 998 pigs) and wean-to-finish (WF; 999 pigs). Serum samples were obtained from the same 20 pigs in each group at weaning and at approximately 50, 85, 110, and 130 days of age. Porcine reproductive and respiratory syndrome virus (PRRSV) and Mycoplasma hyopneumoniae were endemic in this herd. Fisher's r to z analysis was used to determine relationships between serum APPs, serum cortisol, and serum titers for M hyopneumoniae and PRRSV.
Results: There were no production differences between WNF and WF groups. Serum AGP was negatively correlated with weight and PRRS S:P ratio in both groups, but there was no correlation between AGP and M hyopneumoniae titer. In WF pigs, HPT persistently increased, while a sharp increase occurred in WNF pigs on entry into the finisher. Serum HPT was highly correlated with M hyopneumoniae titer in WNF pigs but not WF pigs.
Implications: Under the conditions of this study, increased serum AGP was negatively correlated with body weight, suggesting that an activated cellular immune response is a detriment to growth rate. Further investigations are needed to determine whether these or other APPs are reliable predictors of disease status in swine.
Keywords:  swine, nursery,
wean-to-finish, acute phase proteins, cortisol
swine, nursery,
wean-to-finish, acute phase proteins, cortisol
Received: May 23, 2001
Accepted: January 10, 2002
Acute phase proteins (APPs) have been studied in both human and veterinary medicine to determine if changes in these parameters are indicators of immune stimulation or stress. Acute phase proteins comprise a group of liver-synthesized proteins whose concentration in serum varies in response to injury, infection, inflammation, or common insults, such as stress or diet change.1-4 Changes in APPS can be used to monitor a systemic response to a physiological insult.
It has been suggested that alpha 1-acid glycoprotein (AGP) and haptoglobin (HPT) have potential as physiological indicators of stress, subclinical disease, clinical disease, or combinations of these. Profiling a particular APP might provide diagnostic information that would signal the need for improvement in overall herd health, including changes in management or vaccination scheduling, or initiation of therapeutic regimens.1,3,5-8 The challenge for the veterinary profession is that more than 30 APPs have been identified. To date, there has been little effort dedicated to determining the correlations between APPs and other serological changes in the pig. It was the intent of the authors to document any correlations that might exist, as APPs in swine have not been sufficiently studied to draw any conclusions from changes that might be observed. Serum concentrations of APPs are highly variable in their response to stimuli: serum concentrations of different APPs may decrease or increase in response to the same stimulus.2
Some APPs that increase in response to stress or disease include C-reactive protein (CRP), HPT, fibrinogen, AGP, serum amyloid A, alpha antitrypsin, and ceruloplas-min.3,9,10 In humans, CRP is the most investigated APP. After infections or tissue damage and necrosis, there is up to a thousand-fold increase in CRP concentration.11 In cattle, serum amyloid A and HPT have been recognized as major APPs, with serum amyloid A being the more sensitive of the two. Researchers have found that acute and chronic inflammation in cattle may be differentiated by serum profiles of both serum amyloid A and HPT. Horadagoda et al2 observed that in cattle exposed to Pasteurella hemolytica, the acute inflammatory response could be identified more predictably if serum HPT and serum amyloid A increased together than if HPT increased alone. Other researchers have shown that APPs in cattle increase in response to infections such as bovine leukemia virus, Pasteurella hemolytica, and mastitis.2,4,11
The serum APPs that have been studied most frequently in swine are HPT, AGP, and CRP. Hall et al3 and Eurell et al12 reportedthat the rapid response of HPT to a natural and an experimental challenge (intranasal, 5.4 x 109 colony forming units) with Actinobacillus pleuropneumoniae supports the use of serum HPT as an indicator of subclinical illness and therefore health status and weight gains in pigs. Son et al13 showed that the concentration of AGP in pigs during acute phase response to infection may be high enough to alter protein binding of some drugs, resulting in a decrease in the unbound fraction of drug, thereby decreasing its pharmacological effect. This may help to explain why, in some instances, little or no response to antibiotic therapy is observed. Obtaining additional information with regard to serum APP profiles in swine in a commercial setting will add to our knowledge base regarding the regulation of APPs and their potential use as indicators of an animal's health status. Therefore, the objectives of this study were to evaluate serum concentrations of AGP and HPT in the same pigs over time and to determine whether changes in these APPs were associated with changes in health status and performance.
Materials and methods
Experimental design
Pigs weaned from one sow herd were divided into two groups evaluated in different management systems: a wean-nursery-finish (WNF) group and a wean-to-finish (WF) group.
Approximately 2000 barrows from a 2400-sow, farrow-to-wean breeding stock farm were weaned over a 4-week period beginning on April 1, 1999. Twice a week, 250 pigs were weaned at 11 to 14 days of age and assigned to study groups in accordance with the normal production flow of the system. Nine hundred and ninety eight pigs were weaned into a nursery facility on an isolated site (WNF). Two weeks later, 999 pigs were weaned into a total-slat, double-curtain, wean-to-finish facility (WF) 80 km from the nursery. After 5 weeks (496 pigs) or 7 weeks (495 pigs) in the nursery, the WNF pigs were moved into a grower-finisher facility identical to the wean-to-finish barn and located on the same site. Pen integrity was maintained when the nursery pigs were moved to the grower-finisher site.
After pigs had been assigned to pens, one pig per pen was randomly selected and identified by ear tag for serological sampling (20 pigs per treatment group, 40 pigs total). Serum samples were obtained from the same 40 pigs at weaning and at approximately 50, 85, 110, and 130 days of age. Pigs were marketed from each barn when 175 to 190 pigs had reached an average weight of 118 kg. These pigs were part of a previous study investigating performance and disease status. Their housing and environment have been previously described.14
Pig weights
To validate the effects of the two production systems and gain a better understanding of the weight variability in each pen, all pigs were individually weighed on an electronic digital scale (Tru-Test SR2000; Auckland, New Zealand). Weights were entered directly into a spreadsheet for analysis.
Pigs in the WF group were weighed at weaning (11 to 14 days of age) and at 34 +/- 4, 44 +/- 4, 59 +/- 4, 81 +/- 3, 103 +/- 5, and 119 +/- 5 days of age. Pigs in the WNF group were weighed at weaning (11 to 14 days of age) and at 35 +/- 2 , 46 +/- 5, 60 +/- 3, 74 +/- 3, 94 +/- 3, and 139 +/- 3 days of age.
Average daily gain (ADG), average daily feed intake (ADFI), and feed efficiency (FE) were calculated for the 20 feeders (40 pens) in each barn. The opportunity to weigh pigs by pen ended when the first group of pigs reached a marketable weight of 118 kg and pen integrity was altered.
Feed
The WF and WNF pigs received the same rations throughout this study. A segregated early weaning diet and a transition diet were replaced by a succession of seven different rations.
All pigs were treated with neomycin (Neomix 325; Pharmacia and Upjohn Inc, Kalamazoo, Michigan) in the drinking water for 3 days at weaning. However, the WF pigs were treated with an additional dose of 5 mg per kg BW of neomycin (150 g per proportioner) when they were 33 days old because of an increasing incidence of Escherichia coli diarrhea.
Both groups received medicated feed containing tylosin (Tylan 40; Elanco Animal Health, Indianapolis, Indiana), 100 g per ton (110 mg per kg), for the 3 weeks after proliferative ileitis was diagnosed in the WF pigs at 112 days of age and in the WNF pigs at 126 days of age.
Diagnostic testing
Pathogen exposure at each site was monitored by obtaining repeated serum samples from 20 WNF pigs and 20 WF pigs to observe titer changes for A pleuropneumoniae (types 1, 5, and 7), Mycoplasma hyopneu-moniae, swine influenza virus (SIV), and porcine reproductive and respiratory syndrome virus (PRRSV), and to monitor changes in concentrations of serum HPT, AGP, and cortisol.
Serum samples were analyzed by Biovet Laboratories (St Anthony, Minnesota) using the Exposure Serum Antibody Profiles (ESAP; Biovet Laboratories, St Hyacinthe, Quebec) for all titer changes. The ESAP profile is usually reported as the percent of pigs seropositive for each of the pathogens. However, for the purpose of this study, the results of the serological tests for individual pigs were reported to facilitate statistical evaluation. The tests used in the ESAP were Tween 20 ELISA for SIV, with a sample:positive (S:P) ratio >0.099 considered positive; PRRSV ELISA, S:P ratio >=0.4 considered positive; serum inhibition test for M hyponeumoniae, inhibition >50% considered positive; and Tween 20 ELISA for A pleuropneumoniae types 1, 5, and 7, S:P ratio >0.099 considered positive.
Assays for serum AGP and HPT (ELISA) were performed at the Veterinary Diagnostic Laboratory at the University of Illinois (Urbana, Illinois). These assays have been validated in swine (Jeff Sarno, Cardiotech Services, oral communication, 2001). Serum concentrations of cortisol were determined by the University of Missouri Animal Research Service (Columbia, Missouri) using a single commercially available kit (Diagnostic Products Corporation, Los Angeles, California). This technique has been validated in swine.15 The minimum detectable cortisol level was 2 ng per mL, with a within-assay coefficient of variation of 4.2%.
Tissue samples from pigs that died were submitted to the University of Missouri Veterinary Medical Diagnostic Laboratory (Columbia, Missouri) to determine the causes of death and identify pathogens.
Statistical analysis
All data were analyzed with the pig as the experimental unit
utilizing Statview software.16 All between-group comparisons
between WNF and WF groups at specific time points were made using
an unpaired
t test. All within-group comparisons between two data collection
points were made using a paired t test. Correlation coefficients
(r) are reported on the overall means for each variable for the
WF and WNF groups using Fisher's r to z analysis to describe linear
relationships between two variables within the same group (two
sets of observations from the same pig). Variables used for correlation
comparisons were body weight, M hyopneumoniae titers, PRRS
ELISA S:P ratios, and serum concentrations of cortisol, AGP, and
HPT. Correlation comparisons were made between the parameters
of interest, and the positive or negative change in the linear
relationship of the parameters compared over the same time period
is reported. In all comparisons, P values <.05 were
considered significant.
Results
Health
Over the course of the study, 72 pigs died (7.2%) in the WF group and 44 (4.6%) in the WNF group. Ileitis was the greatest single cause of death in these pigs (4.8% of WF pigs, 2.7% of WNF pigs). Proliferative ileitis was first diagnosed in the WF pigs at 112 days of age and in the WNF pigs at 126 days of age, and Lawsonia intracell-ularis was identified in intestinal mucosa and intestinal content samples collected from several pigs at necropsy over the course of the study period. Diarrhea caused by E coli was first diagnosed at necropsy in the WNF pigs at 17 days of age, and in the WF pigs at 33 days of age. Escherichia coli was isolated from intestinal content samples collected from several pigs at necropsy over the course of the study period.
Serological data
Due to technician error, serum collected from the pigs in both groups at 130 days of age was not tested for antibodies against PRRSV, M hyopneumoniae, SIV, or A pleuropneumoniae.
PRRS ELISA S:P ratios. Mean PRRS ELISA S:P ratios and changes in each group during the study are illustrated in Figure 1. Between 12 and 85 days of age, the mean S:P ratios decreased (P<.001) in the WNF group but did not change significantly in the WF group. Between 85 and 110 days of age, the mean S:P ratios increased (P=.02) in both the WNF and WF groups, and mean S:P ratio at 110 days of age was approximately 3.3-fold greater (P<.001) in the WF group than in the WNF group.
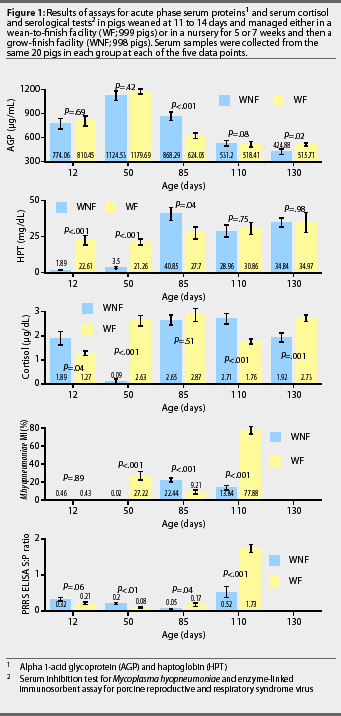
M hyopneumoniae titers. Mean minimum inhibition (MI) titers for M hyopneumoniae and changes in titers for each group during the study are illustrated in Figure 1. Between 12 and 50 days of age, mean MI titer decreased (P<.01) in the WNF group, and increased (P<.001) in the WF group. Between 50 and 85 days of age, mean MI titer increased (P<.001) in the WNF group and decreased in the WF group (P<.01). Between 85 and 110 days of age, mean MI titer decreased by a factor of 1.6 in the WNF group (P <.001), while increasing by a factor of 8.5 in the WNF group (P<.001). As a result, the mean MI titer was approximately 5.6-fold greater (P<.001) in the WF group than in the WNF group at 110 days of age. The maximum number of M hyo-pneumoniae-positive pigs was observed at 85 days of age for the WNF group and at 110 days of age for the WF group.
SIV titers. The percent of pigs seropositive for SIV at 110 days of age was higher (P<.01) for WNF pigs (four of 18 pigs seropositive) than for WF pigs (none of 20 pigs seropositive). There were no detectable titers at other time points.
A pleuropneumoniae titers. All tested pigs were seronegative for A pleuropneumoniae types 1, 5, and 7 at 12, 50, 85, and 110 days of age.
Growth Parameters
There were no differences in ADG and FE between WNF and WF groups. There were no differences in ADG and FE between the WNF pigs that stayed in the nursery for 5 weeks and those that stayed in the nursery for 7 weeks.14
Acute phase proteins and cortisol
AGP. Mean serum concentration of AGP for the two groups
and changes in concentration during the study are shown in
Figure 1. Mean serum AGP increased (P<.03) between 12
and 50 days of age in both the WNF and WF groups. Maximum serum
concentration of AGP was observed at 50 days of age for both groups.
HPT. Mean serum concentration of HPT for the two groups and changes in concentration during the study are shown in Figure 1. Mean serum HPT increased (P=.02) in both groups between 12 and 130 days of age. At 85 days of age, mean serum HPT was 1.5 fold greater (P=.04) in the WNF group than in the WF group. By 110 days of age, serum concentration of HPT had declined (P=.06) in the WNF group, but remained relatively constant (P =.61) in the WF group. Maximum serum concentration of HPT was observed at 85 days of age for the WNF and at 130 days of age for the WF group.
Serum cortisol. Mean serum cortisol for the two groups, and changes in concentration during the study, are shown in Figure 1. Between 12 and 50 days of age, serum cortisol decreased (P<.001) in the WNF group and increased (P<.001) in the WF group. Between 50 and 85 days of age, serum cortisol increased (P<.001) in the WNF group but did not change in the WF group (P>.05). Serum cortisol did not change in the WNF group between 85 and 130 days of age, but tended to decline (P=.06) in the WF group between 85 and 110 days of age. Maximum serum concentration of cortisol was observed at 85 days of age for the WF group and at 110 days of age for the WNF group.
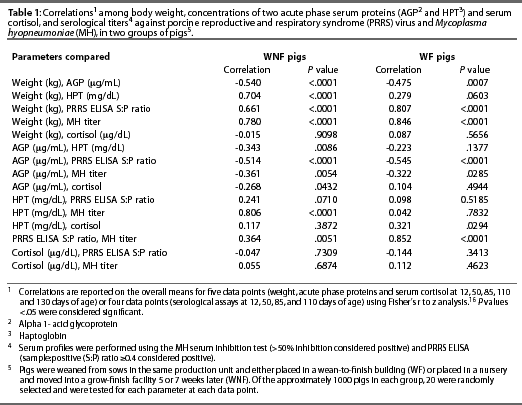
Correlations
Correlations of overall means for the five data points for the serum cortisol and APPs and body weight means, and for the four data points for the serological assays, are shown in Table 1.
Discussion
In a previously reported companion study, there were no differences detected in the ADG, ADFI, or FE between the WNF and WF groups.14 It was our hope to quantify and document differences in immunological parameters between a WF and a WNF system. It has been demonstrated that APPs rise in pigs in response to cyto-kines.17 However, in this study, the use of APPs as indicators of immune activation has proven to be challenging due to the variability of the results.
Serum AGP in both the WNF and the WF groups tended to peak when the pigs were 7 weeks of age, then returned to previously reported values for young adult swine.6 The upper normal limit of serum AGP is reported to be 500 mg per mL for swine 5 to 10 months of age.6 Itoh et al6 found that serum AGP increased in response to concurrent infection with M hyopneu-moniae and A pleuropneumoniae in conventionally raised pigs. In these pigs, serum AGP gradually returned to normal levels, although lesions of chronic pneumonia were identified in most of the animals at slaughter. The authors concluded that an increase in serum AGP is associated with acute rather than chronic infection. Additionally, these investigators reported that in a group of SPF pigs (in which basal AGP levels were lower than those in conventionally raised pigs), serum AGP did not rise above normal levels even in pigs seropositive for M hyopneumoniae.6 In the present study, serum AGP concentration did not increase with increasing M hyopneumoniae MI titer, which is consistent with the results reported by Itoh et al.6
An increase in serum AGP concentrations may be considered an indicator of intracellular communication, suggesting an increase in the cellular immune response.10 In both the WNF and WF groups, serum AGP concentration and PRRS ELISA S:P ratio were negatively correlated, suggesting that when the cellular immune response is active, there may be suppression or limitation of the humoral immune response associated with circulating PRRS virus. A negative correlation between AGP and weight, as observed in this study, suggests that an active cellular immune response adversely affects nutrient accretion and growth rate. Itoh et al6 noted that in pigs chronically exposed to a pathogen, both disease and stressors such as weaning may contribute to increases in AGP.
The gradual increases in serum HPT concentrations in the WF group suggest that these pigs were being chronically stressed throughout the testing period. In contrast, a dramatic increase in HPT was observed in the WNF group in the sample collected when they were 85 days of age. This sudden increase in HPT may reflect the stress associated with movement of the pigs from the nursery to the grow-to-finish facility, where they were exposed to chilling (room temperature 24°C initially) and a large pig space for their size, and the additional stress of finding feed and water in the new environment. In a study by Francisco et al,18 ADG was better in pigs with limited pen space, higher pig density, and warmer room temperature compared to pigs housed with a greater amount of pen space, lower pig density, and a cooler room temperature.In the present study, differences in APPs between the WF and WNF groups were not paralleled by performance differences.
Serum HPT concentrations increased in both groups during the study, and were highly correlated with M hyopneumoniae titers in the WNF group. Hall et al3 reported a rapid response of HPT after either a natural or an experimental challenge with A pleuropneumoniae, and concluded that serum HPT is an appropriate indicator of subclinical illness and health status in pigs.
Serum concentrations of AGP and HPT did not correlate to any change in serum cortisol concentrations, although there was a trend for serum concentrations of HPT to increase at the same time as cortisol concentrations in both the WNF and WF groups. Although serum concentrations of HPT for the WF and WNF pigs were notably different at weaning, cortisol levels were not. However, in the WNF pigs, the sharp increase in HPT between 50 and 85 days of age, when these pigs were adjusting to being moved from the nursery to the finisher, was associated with a similar increase in mean cortisol concentration. In the WF group, serum cortisol had increased between 12 and 50 days of age, before M hyopneumoniae titers and PRRS S:P ratios increased in these pigs, and while serum cortisol levels were decreasing in the WNF pigs. The early increase in serum cortisol in the WF pigs might be attributed to stress. At this time, the WF pigs had been weaned and placed in a new wean-to-finish building, where they were allowed 0.70 m2 per pig with black mats and heat lamps providing zone heating. In this type of building, there is a possibility for chilling of the newly weaned pigs. Cold stress may have been responsible for at least part of the observed increase in serum cortisol concentrations during this period. We speculate that the conventional nursery room, warmed to 29°C, minimized chilling in the WNF pigs. This implies that the environment in the conventional nursery was more suited to the age and size of the early-weaned pig than the WF facility in this study.
Implications
- Serum AGP concentrations of pigs in two management systems were negatively correlated with overall body weights, suggesting that an activated cellular immune response is a detriment to growth.
- Serum HPT may be of value as an indicator of stress in swine herds.
- A combination of serum HPT and serum cortisol concentrations may be a more reliable indicator of disease status or stress in pigs than either parameter alone.
- Further investigations are needed to determine whether serum cortisol, APPs, or a combination of both are reliable predictors of disease status in swine herds.
References - refereed
1. Burger W, Fennert EM, Pohle M, Wesemeier H. C-Reactive Protein-a characteristic feature of health control in swine. J Vet Med. 1992;A39:635-638.
2. Horadagoda NU, Knox KMG, Gibbs HA, Reid SWJ, Horadagoda A, Edwards SER, Eckersall PD. Acute phase proteins in cattle: discrimination between acute and chronic inflammation. Vet Rec. 1999;144:437-441.
3. Hall WF, Eurell TE, Hanson RD, Herr LG. Serum haptoglobin concentration in swine naturally or experimentally infected with Actinobacillus pleuropneumoniae. JAVMA. 1992;201:1730-1733.
4. Nagahata H, Taguchi K, Noda H. Preliminary studies on the acid soluble glycoproteins in serum and their diagnostic value for acute inflammatory disease in cattle. Vet Res Comm. 1989;13:257-263.
5. Eckersall PD, Saini PK, McComb C. The acute phase response of acid soluble glycoprotein, alpha 1-acid glycoprotein, ceruloplasmin, haptoglobin and C-reactive protein in the pig. Vet Immunol Immuno-pathol. 1996;51:377-385.
6. Itoh H, Tamura K, Izumi M, Motoi Y, Kidoguchi K, Funayama Y. The influence of age and health status on the serum alpha 1-acid glycoprotein level of conventional and specific pathogen-free pigs. Can J Vet Res. 1993;57:74-78.
8. Wiedmeyer CE, Solter PF, Francisco CJ, Hall WF, Hoffman WE. Cross-reactivity of an automated human haptoglobin immunoturbidimetric assay for detection of haptoglobin in swine serum. J Vet Diag Invest. 1999;11:295-297.
9. Kushner I, Gewurz H, Benson MD. C-reactive protein and the acute phase response. J Lab Clin Med. 1981;97:739-749.
10. French T. Acute phase proteins. In: The Clinical Chemistry of Laboratory Animals. 1st ed. New York: Pergamon Press, Inc. 1989;201-235.
11. Connor JG, Eckersall PD, Wiseman A, Bain RK, Douglas TA. Acute phase response in calves following infection with Pasteurella haemolytica, Ostertagia ostertagi and endotoxin administration. Res Vet Sci. 1989;47:203-207.
12. Eurell TE, Bane DP, Hall WF, Schaeffer DJ. Serum haptoglobin concentration as an indicator of weight gain in pigs. Can J Vet Res. 1992;56: 6-9.
13. Son DS, Hariya S, Shimoda M, Kokue E. Contributions of alpha 1-acid glycoprotein to plasma binding of some basic antimicrobials in pigs. J Vet Pharmocol Ther. 1996;19:176-183.
15. Daniel JA, Keisler DH, Sterle JA, Matteri RL, Carroll JA. Birth by caesarian alters postnatal function of the hypothalamic-pituitary-adrenal axis in young pigs. J Anim Sci. 1999;77:742-749.
16. Statview. 2nd ed. Cary, North Carolina: Statistical Analysis Systems Institute, 1998.
17. Williams NH, Stahly TS, Zimmerman DR. Effect of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. J Anim Sci. 1997;75:2481-2496.
References - non refereed
7. Clarke JJ, Flahive R, Hassinger II, WJ, Parsons TD. Acute phase protein levels during normal productive cycle of the sow. Proc AASP. Des Moines, Iowa. 1998;75.
