Diagnostic notes |
Non refereed |
Update on postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome diagnostics
Joaquim Segalés, DVM, PhD, Diplomate ECVP
Centre de Recerca en Sanitat Animal (CReSA) - Departament de Sanitat i d'Anatomia Animals, Facultat de Veterinària, Universitat Autònoma de Barcelona, 08193 Bellaterra, Barcelona (Spain);
E-mail: joaquim.segales@uab.esSegalés, J. Update on postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome diagnostics. J Swine Health Prod. 2002;10(6):277-281.
Postweaning multisystemic wasting syndrome (PMWS) has been a very controversial issue since its first description in 1996 by Harding.1 The controversy has focused around three main points: PMWS is clinically similar to the respiratory form of porcine reproductive and respiratory syndrome (PRRS);1 a porcine circovirus, which was considered non-pathogenic in swine,2,3 is present in the tissues of affected pigs;4,5 and it is difficult to consistently reproduce PMWS in experimental models with an inoculum containing porcine circovirus alone.6-12 However, these controversial points were recently resolved. Postweaning multisystemic wasting syndrome has been detected in herds free of PRRS virus (PRRSV), and the disease can be clearly differentiated from PRRSV infection.13-15 A new virus, porcine circovirus type 2 (PCV2), has been described,16-18 which is different from the virus associated with the continuous pig kidney cell line (PK-15 ATCC CCL-33) and known as porcine circovirus type 1 (PCV1). The most recent knowledge indicates that PMWS is a multifactorial disease in which PCV2 is pivotal; ie, PCV2 is strictly necessary to cause PMWS but, in most cases, not sufficient to trigger the clinical disease.6,11,12,19 Recently, Koch's postulates have been fulfilled using only PCV2 isolated from cell culture.8,10,20,21
Porcine circovirus type 2 is now accepted as the etiological agent of PMWS, which has been a serious problem in many parts of the world either alone or in combination with other diseases. Diagnosis of the disease is important to establish proper prevention and control methods. Therefore, this paper reviews methods for approaching the diagnosis of PMWS and of porcine dermatitis and nephropathy syndrome (PDNS), another clinico-pathological condition which has been associated with PCV2 infection.
PWMS diagnosis: useful diagnostic tests
Recent data have shown that PCV2 occurs worldwide and that herd seroprevalence is very high (close to 100%) in most countries where serology has been performed. Since PCV2 is present in herds with and without PMWS,22-24 detection of PCV2 is inadequate as the sole criterion for diagnosing PMWS.
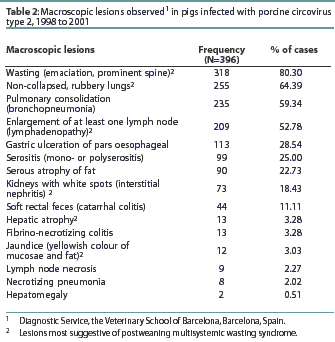
Sorden25 accurately defined PMWS in an individual or group of pigs by three well-defined diagnostic criteria (Table 1) illustrated in Figures 1 through 4. This case definition does not exclude the presence of concurrent diseases. In fact, in a high percentage of cases, PMWS occurs in combination with other clinical conditions, which implies that an overall herd diagnosis (diagnostic approach for all diseases compatible with the clinical signs observed in the herd) is needed to reach an accurate diagnosis of the herd problem. Since other swine diseases may cause wasting, dyspnea, or similar macroscopic lesions,26 clinical signs or gross lesions are only indicative of PMWS (Table 2). However, infection with PCV2 is by definition present in PMWS cases.
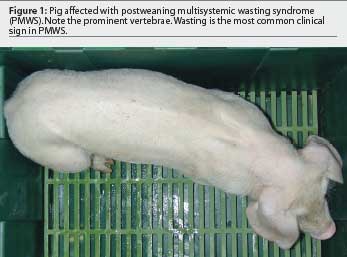
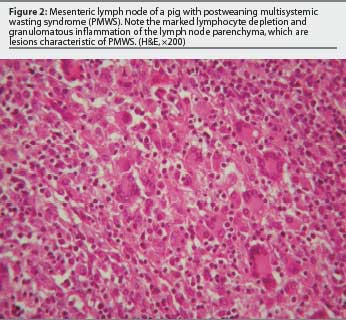
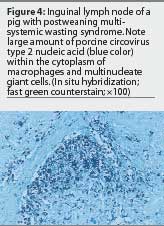
Although several techniques are available to detect PCV2 within lesions characteristic of PMWS, immunohistochemistry for detection of antigen and in situ hybridization (ISH) for detection of nucleic acid have been used most frequently. A very high correlation has also been established between the severity of microscopic lesions in lymphoid tissues and the amount of PCV2 detected within them.4,27,28 The question remains how to interpret cases with mild PMWS-like lesions and very low levels of PCV2. On the one hand, one must be cautious in basing a diagnosis on the laboratory findings of just one pig. On the other hand, laboratory observations must be interpreted in consonance with the evolution of the clinical course of the disease. If mild lesions containing minimal PCV2 are the predominant findings, three possible interpretations can be made. First, the pigs may be suffering from a subclinical PCV2 infection; if clinical signs are similar to those of PMWS, diagnostic tests for other microorganisms should be applied. Secondly, the pigs may be in the initial phases of PMWS; if the analyses have been performed too early in the course of the disease, a second set of samples should be submitted approximately 1 week later. Finally, the pigs may be in the convalescent stage of PMWS; the analyses have been performed too late in the course of the disease, when chronic wasting is the dominant clinical sign.28
To diagnose PMWS, careful selection of pigs and samples for thorough examination is essential. Examination of the entire affected animal (and several pigs if possible) is ideal, as it allows for diagnosis of other diseases besides PMWS. If this is not possible, the most valuable tissues are lymphoid organs (in particular, tonsil, lymph nodes (submit 2 or 3), ileum (for Peyer's patches), and spleen). Although other tissues, such as lung, liver, and kidney, are also useful for detection of PCV2, they are not specific for a diagnosis of PMWS.
Tests insufficient for PWMS diagnosis
A number of important techniques for detection of PCV2 (besides the previously mentioned ones) or antibodies against the virus have been described. Although these techniques are very useful for detection of PCV2 infection or for assessment of the dynamics of the virus infection at the herd level, they alone are insufficient for the diagnosis of PMWS.
In a recent study,29 polymerase chain reaction (PCR) was the most sensitive technique for detecting PCV2, but, compared with ISH, there was less correlation between detection of PCV2 nucleic acid in serum or inguinal lymph nodes and observation of the typical microscopic lesions of PMWS. Thus, PCR is the best technique if the objective is to detect the virus. However, since PCV2 is widespread in herds with and without PMWS, PCR alone is inadequate for diagnosis of the syndrome. In fact, PCR profiles from herds with and without disease may show very similar dynamics of viral circulation. On the other hand, PCR may be a very useful technique for testing potential routes of viral excretion: PCV2 has been detected in nasal, ocular, bronchial, and tonsillar secretions, and in urine, semen, and feces.19,29,30 One investigator has suggested that the greater the number of positive routes of excretion, the higher the probability that the pig had PMWS.31
Several serological techniques for detecting PCV2 antibodies have also been developed.22,23,32,33 Seroconversion to PCV2, measured by an immunoperoxidase monolayer assay (IPMA) or ELISA, shows a pattern typical of viral infections, with colostral antibodies declining during the lactation and nursery phases, the lowest antibody levels at the end of the nursery phase, and active seroconversion of almost all pigs during the grower phase.14,34 In herds with PMWS, mortality is clearly associated with PCV2 seroconversion and a high percentage of viremic pigs.14,35 However, a similar seroconversion pattern may also be found in herds without PMWS.35 In another study, an ELISA test detecting antibodies against the open reading frame 2 protein32 was used to demonstrate that a significantly higher percentage of 13-week-old pigs from herds affected with PMWS were seropositive compared to age-matched pigs from non-affected herds.36 Such epidemiological data are of little value when the objective is to diagnose PMWS in a single herd.
Although other PCV2 detection techniques, such as virus isolation and indirect immunofluorescence, have also been developed,37 these techniques are useful primarily for research studies rather than clinical diagnosis.
PDNS diagnosis
Porcine dermatitis and nephropathy syndrome (PDNS) was first described in the United Kingdom in 1993.38 Since then, countries in Europe, North and South America, Oceania, and Africa have described this condition, suggesting a worldwide distribution.
The case definition for PDNS is relatively simple39-41 and includes two main criteria (Table 3), with typical lesions illustrated in Figures 5 and 6. A small percentage of PDNS cases are considered atypical, without macroscopic skin lesions or with no or minimal kidney lesions. Microscopic lesions characteristic of PDNS occur in these atypical cases.

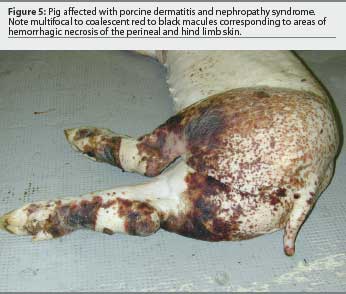
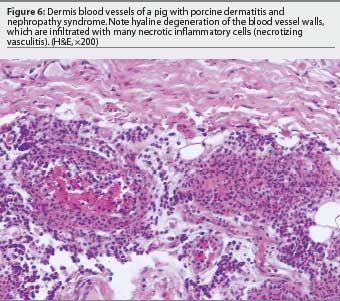
The low prevalence of PDNS in most swine herds (<0.5%) may help in the diagnosis. However, in herds where the prevalence of disease is unusually high (>1%), an accurate differential diagnosis should be established. Other diseases and conditions associated with red discoloration of the skin include classical and African swine fever, swine erysipelas, septicemic salmonellosis, Actinobacillus suis infection, porcine stress syndrome, transit erythema (eg, due to urine-soaked floors or chemical burns), and other causes of bacterial septicemia. In addition, diseases such as classical and African swine fever and septicemic salmonellosis cause kidney lesions similar to those observed in PDNS. As a result, PDNS must be differentiated primarily from classical swine fever in Europe and from septicemic salmonellosis in North America.
The microscopic lesions of PDNS and concurrent detection of immunoglobulin and complement factors in the damaged vessels and glomeruli have suggested a type III hypersensitivity reaction as the possible pathogenic mechanism for PDNS.39,42 It is possible that PDNS is triggered by a wide spectrum of factors, including drugs, chemicals, food allergens, endogenous antigens, and infectious agents.43 It is presently unknown which antigen is associated with this condition.
Mild to moderate lymphocyte depletion and granulomatous inflammation in lymphoid tissues (lesions similar to those observed in PMWS) occur in most pigs suffering from PDNS; these observations have suggested the hypothesis that PCV2 might also be associated with PDNS.40 When large numbers of pigs are examined, PCV2 has been detected by ISH, immunohistochemistry, or both in a high percentage of pigs with PDNS.44-46 Furthermore, PDNS has been associated with PCV2 in Spain, France, Belgium, the United Kingdom, the Netherlands, Argentina, South Korea, Canada, and the United States. Antigen or nucleic acid of PCV2 or both are detected only in very small amounts in pigs with PDNS compared to those with PMWS. Moreover, viral antigen and nucleic acid are absent from the vascular and glomerular lesions of pigs with PDNS.
The current association between PCV2 and PDNS has been established on the basis of circumstantial results and not on a cause-effect relationship. Due to the worldwide enzootic distribution of PCV2, the possibility that the association of PDNS with PCV2 infection is a mere coincidence cannot presently be ruled out. This means that, from a diagnostic point of view, PDNS is a condition with very specific microscopic lesions, and detection of PCV2 is currently not included as a diagnostic criterion.
Acknowledgements
The author deeply appreciates the critical revision of the manuscript by Dr Maria Calsamiglia (Centre de Recerca en Sanitat Animal, Barcelona, Spain) and Dr Monica Balasch (Fort Dodge Veterinaria SA, Girona, Spain).
References - refereed
2. Tischer I, Mields W, Wolff D, Vagt M, Griem W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch Virol. 1986;91:271-276.
3. Allan GM, McNeilly F, Cassidy JP, Reilly GA, Adair B, Ellis WA, McNulty MS. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet Microbiol. 1995;44:49-64.
5. Segalés J, Sitjar M, Domingo M, Dee S, Del Pozo M, Noval R, Sacristan C, De las Heras A, Ferro A, Latimer KS. First report of post-weaning multisystemic wasting syndrome in pigs in Spain. Vet Rec. 1997;141:600-601.
6. Allan GM, Kennedy S, McNeilly F, Foster JC, Ellis JA, Krakowka SJ, Meehan BM, Adair BM. Experimental reproduction of severe wasting disease by co-infection of pigs with porcine circovirus and porcine parvovirus. J Comp Pathol. 1999;121:1-11.
7. Balasch M, Segalés J, Rosell C, Domingo M, Mankertz A, Urniza A, Plana-Durán J. Experimental inoculation of conventional pigs with tissue homogenates from pigs with post-weaning multisystemic wasting syndrome. J Comp Pathol. 1999;121:139-148.
8. Kennedy S, Moffett D, McNeilly F, Meehan B, Ellis J, Krakowka S, Allan GM. Reproduction of lesions of postweaning multisystemic wasting syndrome by infection of conventional pigs with porcine circovirus type 2 alone or in combination with porcine parvovirus. J Comp Pathol. 2000;122:9-24.
9. Magar R, Larochelle R, Thibault S, Lamontagne L. Experimental transmission of porcine circovirus type 2 (PCV2) in weaned pigs: a sequential study. J Comp Pathol. 2000; 123:258-269.
10. Harms PA, Sorden SD, Halbur PG, Bolin S, Lager K, Morozov I, Paul PS. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and PRRSV. Vet Pathol. 2001;38:528-539.
11. Krakowka S, Ellis JA, McNeilly F, Ringler S, Rings DM, Allan G. Activation of the immune system is the pivotal event in the production of wasting disease in pigs infected with porcine circovirus-2 (PCV-2). Vet Pathol. 2001;38:31-42.
12. Rovira A, Balasch M, Segalés J, Garcia L, Plana-Duran J, Rosell C, Ellerbrock H, Mankertz A, Domingo M. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J Virol. 2002;76:3232-3239.
13. Madec F, Eveno E, Morvan P, Hamon L, Blanchard P, Cariolet R, Amenna N, Morvan H, Truong C, Mahé D, Albina E, Jestin A. Post-weaning multisystemic wasting syndrome (PMWS) in pigs in France: clinical observations from follow-up studies on affected farms. Livest Prod Sci. 2000;63:223-233.
14. Rodriguez-Arrioja GM, Segalés J, Calsamiglia M, Resendes AR, Balasch M, Plana-Duran J, Casal J, Domingo M. Dynamics of porcine circovirus type 2 infection in a herd of pigs with postweaning multisystemic wasting syndrome. Am J Vet Res. 2002;63:354-357.
15. Segalés J, Calsamiglia M, Rosell C, Soler M, Maldonado J, Martin M, Domingo M. Porcine reproductive and respiratory syndrome virus (PRRSV) infection status in pigs naturally affected with post-weaning multisystemic wasting syndrome (PMWS) in Spain. Vet Microbiol. 2002;85:23-30.
16. Hamel AL, Lin LL, Nayar GPS. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J Virol. 1998;72:5262-5267.
17. Allan GM, McNeilly F, Meehan BM, Kennedy S, Mackie DP, Ellis JA, Clark EG, Espuña E, Saubi N, Riera P, Botner A, Charreyre CE. Isolation and characterisation of circoviruses from pigs with wasting syndromes in Spain, Denmark and Northern Ireland. Vet Microbiol. 1999;66:115-123.
18. Mankertz A, Domingo M, Folch JM, LeCann P, Jestin A, Segalés J, Chmielewicz B, Plana-Duran J, Soike D. Characterisation of PCV-2 isolates from Spain, Germany and France. Virus Res. 2000;66:5-77.
19. Krakowka S, Ellis JA, Meehan B, Kennedy S, McNeilly F, Allan G. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet Pathol. 2000;37:254-263.
20. Bolin SR, Stoffregen WC, Nayar GP, Hamel AL. Postweaning multisystemic wasting syndrome induced after experimental inoculation of cesarean-derived, colostrum-deprived piglets with type 2 porcine circovirus. J Vet Diagn Invest. 2001;13:185-194.
22. Rodríguez-Arrioja GM, Segalés J, Balasch M, Rosell C, Quintana J, Folch JM, Plana-Durán J, Mankertz A, Domingo M. Serum antibodies to porcine circovirus type 1 (PCV-1) and type 2 (PCV-2) in pigs with and without postweaning multi-systemic wasting syndrome (PMWS). Vet Rec. 2000;146:762-764.
23. Walker IW, Konoby CA, Jewhurst VA, McNair I, McNeilly F, Meehan BM, Cottrell TS, Ellis JA, Allan GM. Development and application of a competitive enzyme-linked immunorbent assay for the detection of serum antibodies to porcine circovirus. J Vet Diagn Invest. 2000;12:400-405.
27. Rosell C, Segalés J, Plana-Durán J, Balasch M, Rodríguez-Arrioja GM, Kennedy S, Allan GM, McNeilly F, Latimer KS, Domingo M. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J Comp Pathol. 1999;120:59-78.
28. Quintana J, Segalés J, Rosell C, Calsamiglia M, Rodríguez-Arrioja GM, Chianini F, Folch JM, Maldonado J, Canal M, Plana-Durán J, Domingo M. Clinical and pathological observations of pigs with postweaning multisystemic wasting syndrome. Vet Rec. 2001;149:357-361.
29. Calsamiglia M, Segalés J, Quintana J, Rosell C, Domingo M. Detection of porcine circovirus types 1 and 2 in serum and tissue samples of pigs with and without postweaning multisystemic wasting syndrome. J Clin Microbiol. 2002;40:1848-1850.
30. Hamel AL, Lin LL, Sachvie C, Grudeski E, Nayar GP. PCR detection and characterization of type-2 porcine circovirus. Can J Vet Res. 2000;64:44-52.
33. Nawagitgul P, Harms PA, Morozov I, Thacker BJ, Sorden SD, Lekcharoensuk C, Paul PS. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin Diagn Lab Immunol. 2002;9:33-40.
37. Allan GM, Ellis JA. Porcine circoviruses: a review. J Vet Diagn Invest. 2000;12:3-14.
39. Hélie P, Drolet R, Germain MC, Bourgault A. Systemic necrotizing vasculitis and glomerulonephritis in grower pigs in southwestern Quebec. Can Vet J. 1995;36:150-154.
40. Segalés J, Piella J, Marco E, Mateu-de-Antonio EM, Espuña E, Domingo M. Porcine dermatitis and nephropathy syndrome in Spain. Vet Rec. 1998;142:483-486.
41. Thibault S, Drolet R, Germain MC, D'Allaire S, Larochelle R, Magar R. Cutaneous and systemic necrotizing vasculitis in swine. Vet Pathol. 1998;35:108-116.
42. Sierra MA, De Las Mulas JM, Molenbeek RF, Van Maanen C, Vos JH, Quezada M. Porcine immune complex glomerulonephritis dermatitis (PIGD) syndrome. Euro J Vet Pathol. 1997;3:63-70.
43. Drolet R, Thibault S, D'Allaire S, Thomson JR, Done SH. Porcine dermatitis and nephropathy syndrome (PDNS): An overview of the disease. Swine Health Prod. 1999;7:283-285.
45. Rosell C, Segalés J, Ramos-Vara JA, Folch JM, Rodríguez-Arrioja GM, Duran CO, Balasch M, Plana-Durán J, Domingo M. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet Rec. 2001;146:40-43.
46. Choi C, Chae C. Colocalization of porcine reproductive and respiratory syndrome virus and porcine circovirus 2 in porcine dermatitis and nephrology syndrome by double-labeling technique. Vet Pathol. 2000;38:436-441.
References - non refereed
1. Harding JC. Post-weaning multisystemic wasting syndrome: preliminary epidemiology and clinical findings. Proc Western Canada Assoc Swine Pract. 1996;21.
4. Clark E. Post-weaning multisystemic wasting syndrome. Proc AASP. Quebec, Canada. 1997:499-501.
21. Allan GM, McNeilly F, Meehan B, Kennedy S, Johnston D, Ellis J, Krakowka S, Fossum C, Wattrang E, Wallgren P. Reproduction of PMWS with a 1993 Swedish isolate of PCV-2. Vet Rec. 2002;150:255-256.
24. Sánchez R, Nauwynck H, Pensaert M. Serological survey of porcine circovirus 2 antibodies in domestic and feral pig populations in Belgium. Proc Eur Soc Vet Virol PMWS. 2001:122.
26. Harding JCS, Clark EG. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 1997;5:201-203.
31. Calsamiglia M, Sibila M, Segalés J, Rosell C, Domingo M. Detection of porcine circovirus type 2 in different routes of excretion: possible transmission routes and correlation with presence of postweaning multisystemic wasting syndrome characteristic lesions. Proc 17th IPVS Congress. Ames, Iowa. 2002:396.
32. Blanchard P, Mahé D, Cariolet R, Truong C, Le Dimma M, de Boisseson C, Rose N, Eveno E, Madec F, Jestin A. Detection of porcine circovirus type 2 (PCV2) specific antibodies in post-weaning multisystemic wasting syndrome (PMWS) studies. Proc Eur Soc Vet Virol PMWS. 2001:104.
34. Harding JC, Clark EG, Ellis JA. The clinical expression of porcine circovirus. Proc Allen D Leman Swine Conf. 1999:252-254.
35. Sibila M, Calsamiglia M, Segalés J, Jestin A, Domingo M. Detection of porcine circovirus type 2 genome in nasal swabs and serum samples from naturally infected pigs using polymerase chain reaction. Proc Eur Soc Vet Virol PMWS. 2001:96.
36. Rose N, Larour G, Le Diguerher G, Blanchard P, Le Dimma M, Eveno E, Jolly JP, Jestin A, Madec F. Serological profile of porcine circovirus type 2 (PCV2) in PMWS affected and non-affected farms. Proc Eur Soc Vet Virol PMWS. 2001:117.
38. Smith WJ, Thomson JR, Done S. Dermatitis/nephropathy syndrome of pigs. Vet Rec. 1993;132: 47.
44. Allan GM, McNeilly E, Kennedy S, Meehan B, Moffett D, Malone F, Ellis J, Krakowka S. PCV-2-associated PDNS in Northern Ireland in 1990. Porcine dermatitis and nephropathy syndrome. Vet Rec. 2000;146:711-712.
