Original research |
Peer reviewed |
Mechanical transmission of enterotoxigenic Escherichia coli to weaned pigs by people, and biosecurity procedures that prevented such transmission
Sandra F. Amass, DVM, PhD, Diplomate ABVP; Patrick G. Halbur, DVM, PhD; Barbara A. Byrne, DVM, PhD, Diplomate ACVIM; Jessica L. Schneider; Carol W. Koons; Nancy Cornick, PhD; Darryl Ragland, DVM, PhD
SFA, BAB, JLS, CWK, DR: National Biosecurity Resource Center, Purdue University School of Veterinary Medicine, VCS/Lynn, 625 Harrison Street, West Lafayette, IN 47907-2026. PGH: Iowa State University Veterinary Diagnostic Laboratory, Ames, IA 50010. NC: Veterinary Microbiology and Preventive Medicine, Iowa State University, Ames, IA 50010.
Amass SF, Halbur PG, Byrne BA, et al. Mechanical transmission of enterotoxigenic Escherichia coli to weaned pigs by people, and biosecurity procedures that prevented such transmission. J Swine Health Prod. 2003;11(2):61-68. Also available as a PDF
Summary
Objectives: To determine whether people can mechanically transmit enterotoxigenic Escherichia coli (ETEC) from infected to susceptible weaned pigs during direct pig contact and to determine biosecurity measures that will prevent such transmission.
Materials and methods: One hundred and twenty-five 19- to 21-day-old weaned pigs, culture-negative for ETEC M1823B, were randomly allocated to six treatment groups housed in five separate isolation rooms. Inoculated Pigs were offered 1.36 x 1010 to 8.92 x 1010 colony forming units of E coli mixed in strawberry gelatin on two occasions. Pen Sentinels were housed with Inoculated Pigs. A caretaker fed pigs, checked waterers, and directlycontacted each group of pigs for 10 minutes daily for 10 consecutive days. The caretaker contacted Inoculated Pigs and moved directly to Direct Sentinels, recontacted Inoculated Pigs, washed hands twice, changed outerwear, then contacted Hand-wash Sentinels. The caretaker then recontacted Inoculated Pigs, showered, changed outerwear, and contacted Shower Sentinels. Non-exposed pigs had a separate caretaker.
Results: Escherichia coli M1823B was isolated from all 20 Inoculated Pigs, all five Pen Sentinels, 20 of 25 Direct Sentinels, and 23 of 25 Hand-wash Sentinels. The 25 Shower Sentinels and 25 Non-exposed Pigs remained culture-negative for M1823B.
Implications: In this study, people mechanically transmitted E coli without extraordinary measures to enhance caretaker contact with pig excretions and secretions beyond that which would occur in a typical pork production unit. Hand washing and donning clean outerwear did not prevent E coli transmission. However, showering and donning clean outerwear did prevent transmission.
Keywords: swine, post-weaning diarrhea, Escherichia coli, transmission, biosecurity
Received: August 12, 2002
Accepted: October 10, 2002
Post-weaning diarrhea in swine caused by enterotoxigenic Escherichia coli (ETEC) may be an important cause of mortality in weaned pigs and has become a frustration in many pork producers' units.1-4 Postweaning E colidiarrhea was reported as the most regular disease problem of "large-scale" pig farms, producing significant losses in weaned pigs.3 The cost of E coli diarrhea in all ages of pigs was estimated at £11.5 million (US$18.4 million) per year for the UK national herd of approximately 800,000 sows.4 Post-weaning diarrhea and death loss caused by E coli emerged as a concern in Ontario in late 1997.2 A Canadian study estimated that mortality resulting from post-weaning E coli diarrhea in a 500-sow herd would cost CAN$20,000 annually.2 Vaccination, medication, optimization of nutrition and sanitation, and minimization of management stressors are common procedures used to control E coli diarrhea in pigs.3,5 However, in some herds, post-weaning diarrhea due to E coli persists despite vaccination, aggressive use of antibiotics, and improved facility sanitation. Consequently, in these herds, other risk factors contributing to the spread of colibacillosis must be considered. In 2000, a group of approximately 20 veterinarians met to discuss the problem of post-weaning E coli diarrhea in the industry. This study was initiated as a result of that meeting, to address the possibility that ETEC is spread by caretakers tracking the pathogen from infected to susceptible groups of pigs. In support of field observations, we have previously shown that transmissible gastroenteritis virus (TGEV) of swine was mechanically spread under experimental conditions in which an investigator moved from TGEV-inoculated pigs to susceptible pigs without using biosecurity procedures. The investigator was in direct contact with pigs and their secretions and excretions for 10 minutes, twice daily, for 2 weeks.6 Similar transmission of E coli is likely, considering that spread of E coli occurs through fecal-oral transmission and some strains survive for 47 days in bovine manure and 21 months in ovine manure under fluctuating environmental conditions.7
The specific objectives of this study were to determine whether people can mechanically transmit ETEC from infected to susceptible weaned pigs during direct pig contact and to determine which biosecurity measures prevent such transmission under the conditions of the study.
Materials and methods
Animals and experimental groups
One hundred and twenty-five 19- to 21-day-old weaned pigs from a single source were used. The source herd had no history of post-weaning diarrhea caused by E coli. Fecal samples from weaned pigs at the source herd, collected prior to the study, were negative for the E coli strain used as inoculum. Moreover, all pigs were sampled on arrival (Day 0) to verify that none carried a lactose-positive organism resistant to the concentrations of nalidixic acid and tetracycline found in the agar, and pigs used in the study were all negative for the challenge strain of ETEC M1823B by fecal culture. Pigs were randomly allocated to one of six treatment groups: Inoculated Pigs, Pen Sentinels, Direct Sentinels, Hand-wash Sentinels, Shower Sentinels, or Non-exposed Pigs (Table 1).
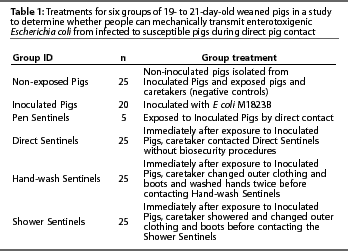
Facilities, environment, and diet
Each group of pigs was maintained in a separate room connected by a common hallway. Pen Sentinels were housed in the same room as Non-exposed Pigs for the first 17 hours of the study to avoid contact with challenge-inoculum. Rooms were 3.81 m x 5.18 m with sealed, epoxy-coated floors and two drains. Pigs were housed in 2.42 x 1.82-m pens with elevated, plastic-coated expanded metal flooring, a 1.21-m long stainless steel nursery feeder, and two nipple waterers. Rooms were HEPA filtered and had negative pressure ventilation. Prior to the study, rooms and equipment were cleaned by pressure washing and then disinfected using a quaternary ammonium compound (Roccal-D Plus; Pharmacia & Upjohn Company, Kalamazoo, Michigan) according to label directions. Swab samples randomly collected from the pen floor, waterer, feeder, and room floor of each room after the disinfectant dried were culturally negative prior to pig entry. The drains under the pig decks were plugged with steel wool and covered with plastic during the study, such that material in the university sewage system could not back up into the rooms. Pigs were fed ad libitum a non-medicated diet formulated for nursery pigs.
Description and administration of E coli inoculum
The inoculum was E coli strain M1823B (kindly donated by Dr Harley Moon), a porcine strain of serotype O157:H43.8 This strain expresses K88 and F41 fimbriae and produces heat labile enterotoxin (LT) as well as heat stable enterotoxin b (Stb). For administration to the Inoculated Pigs, strain M1823B in trypticase soy broth was diluted 1 vol:1 vol in liquid strawberry gelatin (Jell-O gelatin dessert, Strawberry; Kraft Foods Inc, Rye Brook, New York). Pilot studies demonstrated that E coli viability was not affected by a 1 vol:1 vol dilution in Jell-O (S.F. Amass, DVM, PhD, Diplomate ABVP, and J.L. Schneider, unpublished data, 2001).
The inoculum was administered to the Inoculated Pigs on two occasions. The first administration was on the day of arrival (Day 0), approximately 2 hours after fecal samples had been collected and the pigs were allocated to rooms. The inoculum was offered to each individual pig. Twelve of the 20 Inoculated Pigs consumed the challenge inoculum on Day 0 at a dose of 2.23 x 1010 to 8.92 x 1010 colony forming units (cfu) per pig.
On Day 2, 48 hours after the initial challenge inoculation, Inoculated Pigs were individually offered fresh challenge inoculum. Eighteen of 20 Inoculated Pigs consumed the inoculum at a dose of 1.36 x 1010 to 2.04 x 1010 cfu per pig.
As a consequence of this method of inoculation, one of 20 Inoculated Pigs did not consume inoculum, and ten of 20 Inoculated Pigs consumed inoculum on two occasions. The number of times that a pig consumed inoculum was considered irrelevant to the study design, because the pen was the experimental unit. Therefore, as long as one pig in the pen was inoculated successfully, the E coli would presumably infect other pigs, which would act as the source of E coli to be transmitted.
Study design
A single replicate of this study was conducted. All pigs arrived at the isolation facility at the same time (Day 0).
On arrival, a fecal sample was collected from each pig to confirm that all were culture-negative for the E coli inoculum strain. Approximately 2 hours after sampling was completed (Day 0), E coli M1823B was offered to Inoculated Pigs, which were removed to a separate pen in the same room for the inoculation procedure. Seventeen hours after arrival (Day 1), the five Pen Sentinels were placed in the pen with the Inoculated Pigs. On Day 2, 48 hours after the initial challenge inoculation, Inoculated Pigs were again moved to a separate pen in the same room for the second inoculation, to avoid exposing the Pen Sentinels to the inoculum. After inoculation procedures were repeated, the Inoculated Pigs were returned to the pen they shared with the Pen Sentinels.
Starting on Day 1 and continuing for 10 consecutive days, the four groups of sentinel pigs (Table 1) were exposed to the Inoculated Pigs either directly or by exposure to people who contacted the Inoculated Pigs (Table 2).
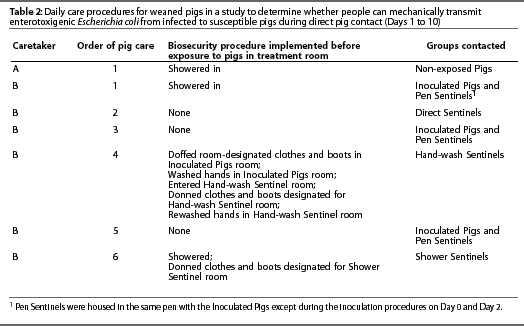
Rectal swabs for fecal culture were collected from all pigs on Days 2, 4, and 7. Pigs that exhibited diarrhea, other than Inoculated Pigs, were removed from the room, humanely euthanized, and sampled for bacterial culture and histopathology. All Inoculated Pigs, sentinel pigs that did not exhibit diarrhea, and Non-exposed Pigs were humanely euthanized and sampled on Day 11.
Personnel procedures
Inoculated and sentinel pigs were cared for by a single caretaker, and Non-exposed Pigs were cared for by a separate caretaker, who was the only person to enter that room during the course of the study. Hallway traffic patterns were delineated such that caretakers did not cross paths. Each caretaker had a designated shower facility. Standard shower facilities with clean and contaminated sides were used. Both caretakers followed the same procedures. Caretakers wore minimal undergarments that did not protrude from coveralls. Immediately after entering a room, each caretaker donned room-designated, short-sleeved cloth coveralls and boots just inside the door. The caretaker for Inoculated and sentinel pigs wore rubber boots and the caretaker for Non-exposed Pigs wore disposable plastic boots.
Caretakers fed pigs from outside the pens, set a timer for 10 minutes, and then climbed into the decks with the pigs. In the decks, caretakers checked waterers and stood in direct contact with pigs for 10 minutes. Pigs were observed for clinical signs of diarrhea, and occurrence of diarrhea was recorded. Both caretakers picked up each pig during sampling on Days 2, 4, and 7. The caretaker for Inoculated Pigs and sentinels picked up pigs in which the act of defecation was not observed, palpated the abdomen, and examined the perineal area for diarrhea. Pigs were also picked up to assist in reading ear tag numbers, and diarrheic pigs were picked up to remove them from the room. Most Inoculated Pigs and sentinels were handled by the caretaker during the observation period. The caretaker for Non-Exposed Pigs rarely handled pigs except during sampling. These procedures were repeated daily for 10 consecutive days. Pigs were cared for in a consistent order, testing increasing levels of biosecurity (Table 2).
Hand-washing procedures were implemented as follows: Prior to hand washing, the caretaker doffed room-designated clothes and boots in the Inoculated Pigs room and then washed hands in that room. The caretaker then entered the Hand-wash Sentinel room, donned room-designated clothes and boots, and rewashed hands. Hand washing consisted of washing hands with soap (Dial antibacterial liquid soap; Dial Corporation, Scottsdale, Arizona) and warm water until organic material was no longer visible, and then drying hands with a paper towel.
Showering consisted of shampooing hair (Equate Herbal Shampoo with rose hips, vitamin E, and jojoba, Vi-Jon Laboratories Inc, St Louis, Missouri) and washing with soap (Body Essence Moisturizing Tangerine Spice Body Wash Bath and Shower Gel, Puretek Corp, San Fernando, California) and warm water until no organic material was visible and then towel-drying. Fingernail brushes were not used during hand washing or showering.
Pig removal procedures
On Days 1 through 10 of the study, non-inoculated pigs exhibiting diarrhea during an observation period were carefully removed (to avoid cross-contamination of other rooms) at the end of that observation period. Briefly, after observation in each room, the caretaker carried each diarrheic pig to the room door and handed it, without touching the investigator, to a separate clean investigator standing in the hallway wearing nitrile gloves, cloth coveralls, and disposable boots. The investigator put the hindquarters of the pig into a disposable plastic boot (which was not reused) to prevent hallway contamination. The investigator carried the pig to the main door of the facility and placed it directly into a covered cart outside the facility. When all the pigs to be euthanized from a single room were in the cart, it was transported to the door of the diagnostic laboratory. The clean investigator then handed pigs to a third investigator who took them into the diagnostic laboratory. Organic material was washed from the cart and it was disinfected (Roccal-D Plus) and transported back to the outside door of the animal housing facility to pick up pigs from the next room. The clean investigator donned fresh nitrile gloves and boots for each group of pigs, and fresh coveralls if coveralls became soiled while carrying pigs. The transport cart never entered the diagnostic laboratory or the animal housing facility. On Day 11, pigs were removed by group from least to most contaminated (ie, Non-exposed Pigs first, Inoculated Pigs last) and transported to the diagnostic laboratory.
Collection of samples and diagnostic evaluation
Caretakers recorded presence or absence of diarrhea in each pig daily.
Rectal swab samples for fecal culture were collected from all pigs at entry (Day 0) and on Days 2, 4, 7, and 11 using sterile swabs in transport media (S/P Brand culturette system; Baxter Diagnostics Inc, Deerfield, Illinois). Additional personnel who had not contacted pigs or E coli M1823B for 5 days prior to the study assisted with sample collection. Each assistant showered in and was assigned to a single room. These personnel did not enter pig pens. The caretaker stood in the pen and manually restrained each pig by holding it over the pen divider. The assistant donned nitrile gloves and clean outerwear, stood outside the pen, and swabbed the pig. The caretaker for Non-exposed Pigs collected all swab samples without assistance. Immediately after collection, swabs were hand-carried to the laboratory.
Rectal swabs were cultured for isolation of E coli with antimicrobial sensitivity patterns characteristic of the challenge isolate. A maximum of six isolates per room were further characterized by detection of pilus and enterotoxin genes using multiplex polymerase chain reaction (PCR), and for agglutination with O157 antisera. Detection of E coli strain M1823B in either the feces or intestines of sentinels was considered evidence of mechanical transmission of the organism. Complete necropsies were performed on all pigs and gross lesions were recorded. Two 1-cm sections of duodenum, jejunum, and ileum were collected and examined microscopically, and observation of villous atrophy, inflamma-tion, and non-E coli organisms was recorded.
Culture of fecal swabs and identification of isolates
Fecal swabs were inoculated on MacConkey agar prepared, according to the manu-facturer's instructions (Difco Laboratories, Detroit, Michigan), with 80 mg per ml nalidixic acid (Sigma-Aldrich, St Louis, Missouri) and 30 mg per ml tetracycline (Sigma-Aldrich). The samples were streaked for isolation and plates were cultured at 37°C for 18 to 24 hours. Colonies that grew on this agar, were lactose-positive, and precipitated bile salts were considered positive for the inoculum strain.
Recovered E coli isolates were screened for virulence genes using a multiplex PCR assay.9 This assay detects the fimbriae F4 (K88), F5 (K99), F6 (987P), F18, F41, heat stable enterotoxins (STa, STb), heat liable enterotoxin, and Shiga toxin 2. Isolates were also confirmed as serotype O157 using a latex agglutination kit (E coli O157 Latex Kit; Oxoid, Blasingstoke, England) according to the manufacturer's instructions.
Statistical analysis
Mean and median times from Day 0 to detection of E coli M1823B in pigs were calculated for comparison of Direct Sentinels and Hand-wash Sentinels. The data for this period was nonparametric, and the Mann Whitney test was used, with P<.05 considered statistically significant. As E coli M1823B was not detected in the Shower Sentinels, this group was not compared to Direct Sentinels and Hand-wash Sentinels. GraphPad InStat version 3.00 for Windows 95 (GraphPad Software, San Diego, California) was used for statistical calculations.
Results
Non-exposed Pigs
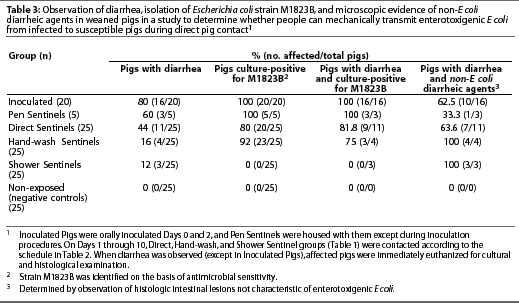
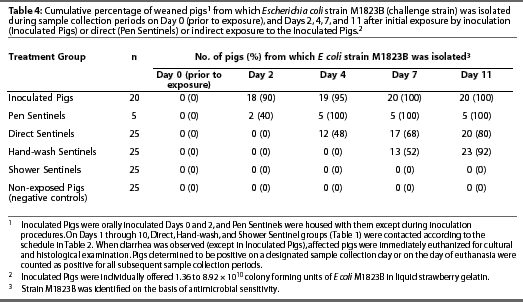
Non-exposed Pigs did not exhibit diarrhea during the study (Table 3) and were culture-negative for E coli strain M1823B at all data collection points (Table 4).
Inoculated Pigs
Escherichia coli strain M1823B (identified on the basis of antimicrobial sensitivity results) was isolated from each Inoculated Pig at one or more data collection points (Table 3). Escherichia coli was first detected on Day 2 (mean +/- SD = 2.35 +/- 1.182 days; median = 2 days) (Table 4). Moreover, isolates from six pigs were further characterized by latex agglutination and multiplex PCR, and all six (100%) were determined to be Escherichia coli strain M1823B.
Many Inoculated Pigs exhibited diarrhea; however, some of these pigs had microscopic lesions associated with other causes of diarrhea (Table 3), including villous atrophy, inflammation, or both in seven pigs; organisms consistent with Cryptosporidium species in two pigs; and villous atrophy, inflammation, and organisms consistent with Cryptosporidium species in one pig.
Pen Sentinels
Escherichia coli strain M1823B (identified on the basis of antimicrobial sensitivity results) was isolated from each Pen Sentinel at one or more data collection points, and isolates were confirmed to be strain M1823B by latex agglutination and multiplex PCR (Table 3). Escherichia coli was first detected on Day 2 (mean +/- SD = 3.2 +/- 1.095 days; median = 4 days) (Table 4).
Diarrhea was observed in only three of the five Pen Sentinels. All three pigs with diarrhea were culture-positive for strain M1823B, but one of them had microscopic intestinal lesions of inflammation (Table 3).
Direct Sentinels
Escherichia coli strain M1823B (identified on the basis of antimicrobial sensitivity results) was isolated from 80% of Direct Sentinels at one or more data collection points (Table 3). Escherichia coli was first detected on Day 4 (mean +/- SD = 6 +/- 2.828 days; median = 4 days) (Table 4). Six of the six isolates tested were confirmed to be strain M1823B by latex agglutination and multiplex PCR.
Diarrhea was observed in only 9 of the 11 Direct Sentinels that were culture-positive for E coli M1823B (Table 3). Seven of these pigs (64%) had microscopic intestinal lesions of villous atrophy, inflamma-tion, or both.
Hand-wash Sentinels
Escherichia coli strain M1823B (identified on the basis of antimicrobial sensitivity results) was isolated from 92% of Hand-wash Sentinels (Table 3). Escherichia coli was first detected on Day 7 (mean +/- SD = 8.565 +/- 2.063 days; median = 7 days) (Table 4). Time from Day 0 to the day of detection of E coli during set sampling periods was longer for Hand-wash Sentinels compared to Direct Sentinels (P=.002). Six of the six isolates tested were confirmed to be strain M1823B by multiplex PCR.
Diarrhea was observed in only four Hand-wash Sentinels, and three of these were culture-positive for E coli M1823B (Table 3). All four pigs exhibiting diarrhea had microscopicintestinal lesions of villous atrophy, inflammation, or both.
Shower Sentinels
Diarrhea was observed in three Shower Sentinels; however, all pigs remained culture-negative for E coli strain M1823B at all data collection points (Tables 3 and 4). Two of the three pigs exhibiting diarrhea had microscopic intestinal lesions of inflammation, and organisms consistent with Cryptospor-idium species were observed in the third pig.
Discussion
To test the mechanical transmission of E coli by people, pigs in direct contact with Inoculated Pigs (Pen Sentinels), pigs in contact with people exposed to Inoculated Pigs (Direct Sentinels), Non-exposed Pigs (negative controls), and two intervention groups (Hand-wash Sentinels and Shower Sentinels) were compared. The Inoculated Pigs group, which were the source of E coli to be transmitted, were directly infected by consumption of E coli strain M1823B or indirectly infected by contact with pen mates who had consumed E coli strain M1823B. These pigs were actively shedding E coli strain M1823B, as evidenced by transmission to Pen Sentinels housed in the same pen. The caretaker for Inoculated and Sentinel Pigs was exposed to Inoculated Pigs immediately before each intervention was used. Exposures were repeated once a day for 10 days such that each intervention was stringently tested.
Microscopic examination of intestine was employed because diarrhea may have multiple etiologies. The villous atrophy and inflammation observed in some pigs are not consistent with ETEC, but could explain alternate causes of diarrhea. The herd of origin of the experimental pigs was clinically negative for TGEV and rotavirus and was serologically negative for antibodies to TGEV (Svanovir; Svanova Biotech, Uppsala, Sweden). Three of the pigs with villous atrophy tested negative for both TGEV and rotavirus by immunohistochemistry. Organisms consistent with Cryptosporidium species were observed on microscopic examination of intestine from some pigs. Thus, in some cases, a pig with diarrhea might have been removed from this trial before exposure to E coli M1823B. This limitation should not have affected overall study results, as the pen was the experimental unit. Use of multiplex PCR in conjunction with bacterial culture provided highly sensitive and more specific diagnostic techniques to confirm colonization of sentinel pigs by our challenge strain of E coli M1823B, despite the presence of other etiologic agents of diarrhea.
The results of this study support the assertion that people can act as mechanical vectors of E coli when moving between groups of infected and susceptible pigs. Moreover, mechanical transmission occurred without extraordinary measures being taken to enhance caretaker contact with pig excretions and secretion beyond that which would occur in a typical pork production unit. A more virulent isolate than that used in this study conceivably would need a smaller dose to cause disease; thus, any transmission is important.
Our results provide evidence that washing hands and donning clean outerwear was insufficient to prevent mechanical transmission of E coli by the caretaker in our study. One reason for the failure of this intervention in our study is that hand washing was not sufficient to remove all E coli-containing organic material from hands of the caretaker, such as deep under the fingernails. The caretaker washed her hands until all visible organic material was removed. However, a fingernail brush was not used during hand washing in this study, and E coli could have been invisibly carried deep under the fingernails. Alternative explanations are that a pig contacted an infectious dose of E coli from contaminated parts of the caretaker such as her face, mouth, neck, arms, or hair, because hand washing did not remove E coli from these body parts. Although due care was taken, the caretaker might have inadver-tently touched a contaminated body part after hand washing. In contrast, showering and donning clean outerwear was sufficient to prevent transmission of E coli this study. Presumably, showering provided a whole-body decontamination procedure that was effective in removing organic material from other exposed areas in addition to the hands. Material carried under fingernails was possibly removed during hair washing. However, time from Day 0 to the day of detection of E coli during set sampling periods was longer for Hand-wash Sentinels compared to Direct Sentinels, suggesting that hand washing and donning clean outerwear decreased the number of organisms carried by the investigator such that it took longer to achieve an infectious dose in sentinel pigs. The infectious dose might have been achieved by repeated contacts with the caretaker until an infectious dose was reached, contact with an infected pen mate in which the delay was the result of time needed for the E coli to replicate within the infected pig and be shed to pen mates, or, more likely, a combination of the two events. The results of this study suggest that hand washing and donning clean outerwear as performed in this study is not a sufficient biosecurity measure to prevent transmission of E coli. Thus, shower-in facilities might be considered for production units that are using some biosecurity measures (eg, isolation and acclimatization facilities, all-in, all-out pig flow with cleaning and disinfection between groups, hand washing, boot changing, and coverall changing before entry), but are still experiencing uncontrollable losses due to E coli.
Previous studies have examined the role of people in mechanical transmission of porcine reproductive and respiratory syndrome virus (PRRSV) and TGEV. In the first study,10 contamination of people was accomplished by having ten people sit in a pen with viremic, PRRSV-inoculated pigs for 1 hour. People handled the pigs as the pigs chewed on the coveralls and rubber boots worn by the participants. Extraordinary measures (beyond those which would be used in a typical pork production unit) were not undertaken to enhance contamination of participants with PRRSV. Five participants then contacted sentinel pigs in the same manner without employing biosecu-rity measures. The remaining five participants showered, donned clean coveralls and boots, and then contacted a second group of sentinel pigs in same manner. Sentinel pigs had separate caretakers and were monitored for clinical signs of PRRSV for 23 days after exposure to contaminated people, at which time sentinel pigs were euthanized and samples were collected for PRRSV isolation and serology. In this study, PRRS viral RNA was detected in saliva and fingernail rinse samples from two of ten people immediately after exposure to PRRSV-inoculated pigs, in a fingernail rinse sample from a third person at 5 hours after exposure to PRRSV-inoculated pigs, and in a nasal swab sample from a fourth person at 48 hours after exposure to PRRSV-inoculated pigs.10 Virus isolationwas not performed in this study; therefore, the viability, infectiousness, and amount of the virus contaminating participants remained unknown. Despite contamination, people did not act as mechanical vectors for PRRSV to sentinel pigs in this study. This study was limited because only a single replication was performed. Moreover, investigators could not prove that PRRSV-inoculated pigs were shedding an infectious dose of PRRSV becauseuninoculated pigs were not housed with inoculated pigs to act as sentinels for transmissibility.
In the second study,11 contamination of personnel was extensive and accomplished by having the investigator don boots and coveralls and spend 1 hour in a room with PRRSV-inoculated pigs biting and licking hands, coveralls, and boots. During the hour, the investigator picked up each of seven PRRSV-inoculated pigs and performed the following tasks: placed the PRRSV-inoculated pig's snout on the dorsal and ventral surface of each hand and rubbed the snout repeatedly across the boots and coveralls; placed each hand in the pig's mouth for 5 seconds; sprayed blood from the PRRSV-inoculated pig over the coveralls, boots, and dorsal and ventral surfaces of hands; and, picked up a handful of fecal material which was spread on the hands, boots and coveralls of the investigator. Thus, extraordinary measures not performed on a typical pork production unit were implemented to contaminate personnel. Investigators then moved to rooms of sentinel pigs after using no biosecurity procedures, washing hands and changing clothes and boots, or showering and changing clothes and boots with and without a 12-hour animal avoidance period. The investigator using no biosecurity procedures moved directly to sentinel pigs without changing clothes, boots, or washing hands. The investigator contacted sentinel pigs for 30 minutes in a similar fashion as stated above, but without exposure to blood or feces of sentinel pigs. Following exposure, the investigator doffed contaminated clothes and boots and left them in the pen with sentinel pigs for 24 hours. Four replications were performed. In this study, PRRSV was detected on the hands of one person in one of four replicates by PCR and swine bioassay. Infection of sentinel pigs by PRRSV was demonstrated in two of four replicates when biosecurity procedures were not used and contaminated coveralls and boots were left in the pen with sentinel pigs. Infection of sentinel pigs was not demonstrated in other treatment groups. One limitation of this study was that the extensive procedures used to contaminate personnel with PRRSV did not reflect conditions on typical pork production units. Thus, isolation of PRRSV from the hands of personnel after such gross contamination was not surprising. The primary limitation of this study was that the investigator using no biosecurity procedures left contaminated outerwear in the pen of sentinel pigs for 24 hours following human exposure. While again not reflective of conditions on a typical pork production unit, this action confounded the examination of human transmission. Specifically, one could not determine if the 30 minutes of human contact was sufficient to transmit PRRSV to sentinel pigs or if the source of PRRSV was the grossly contaminated coveralls and boots that the pigs had presumably chewed on for up to 24 hours. Thus, although both studies found evidence of PRRSV contamination of people after exposure to PRRSV-infected pigs, neither study effectively examined whether people could indeed mechanically transmit PRRSV to susceptible pigs. Without this knowledge, the effectiveness of intervention strategies cannot be assessed, because if people cannot mechanically transmit PRRSV, intervention strategies are not needed.
The single replicate study using TGEV found that hand washing and donning clean outerwear and boots was sufficient to prevent mechanical transmission of TGEV from inoculated pigs to susceptible pigs under experimental conditions.6 In this study, the caretaker moved among groups of pigs using increasing levels of biosecurity. The caretaker had direct contact with TGEV-Inoculated pigs, then used no biosecurity and contacted Direct Sentinels, then washed hands twice and changed outerwear and contacted Hand-wash Sentinels, and, finally, showered and changed outwear before contacting Shower Sentinels. Pigs were contacted by the caretaker in this order for a 10-minute period, twice per day, for 14 days. The caretaker did not recontact TGEV-Inoculated pigs between contacts with Sentinel pigs. Hand-washing procedures used in the TGEV study were identical to those used in the E coli study, and antimicrobial soaps were used in both studies. The sentinel pigs in the TGEV study were seronegative and susceptible to TGEV, but were >=4 weeks old when exposed. Thus, due to their age, these pigs might have been less susceptible to a presumably smaller dose of TGEV transmitted after hand washing, whereas the presumably larger dose of virus transmitted by the investigator when biosecurity procedures were not used was sufficient to cause infection despite the age of the pigs. Hand washing might not have been effective if younger sentinel pigs had been used in the study.
Conflicting results in biosecurity trials are not surprising. We hypothesize that the effectiveness of biosecurity procedures needed to prevent mechanical transmission of pathogens by people is variable due to the vast differences among pathogens and host susceptibilities. For example, the nature (pathogen, commensal), type (Gram-negative bacterium, Gram-positive bacterium, enveloped virus, nonenveloped virus) infectiousness, and contagiousness of the organism must be considered. Moreover, host susceptibility depends on many factors, including, but not limited to age, immunocompetency, vaccination status, genetic predisposition, concurrent illness, stress, environment, management, and nutrition. The frequency of exposure and routes of transmission will also impact the efficacy of biosecurity protocols. In the field, large populations of swine, lack of compliance by personnel, large pathogen loads, and incorrectly performed or inadequate facility sanitation may all confound the efficacy of biosecurity procedures. Consequently, one would not expect a single biosecurity protocol to be efficacious in all cases.
For the above reasons, caution must be taken when interpreting our results, because the study consisted of only a single replicate, and repeatability of our results is unknown. Moreover, the effectiveness of biosecurity interventions tested in this study may not reflect their efficacy under field conditions. The inherent variability in efficacy of biosecurity protocols exemplifies the need for further experimental and field studies to evaluate confounding factors on protocol efficacy. Thus, producers and veterinarians are encouraged to test biosecurity interventions on their farms to determine the most effective and appropriate biosecurity measures for their specific situation.
Finally, the presence of E coli strain M1823B in pigs without diarrhea emphasizes the importance of strict personal hygiene to prevent mechanical transmission of pathogens. Many times, biosecurity procedures are implemented on farms in response to a disease outbreak. Biosecurity protocols must be implemented in a proactive manner, because people may mechanically transmit organisms among groups of pigs before clinical signs become apparent.
Implications
- People can mechanically transmit E coli among groups of pigs.
- Under the conditions of this study, hand washing and donning clean outerwear delayed, but did not prevent, the mechanical transmission of E coli by the caretaker.
- Under the conditions of this study, showering and donning clean outerwear prevented the mechanical transmission of E coli by the caretaker.
- Subclinical colonization of pigs with
- E coli emphasizes the need for proactive biosecurity procedures.
Acknowledgments
The National Pork Board provided all funding for this project. We would like to thank Cheryl Anderson, Tom Brown, Brooks Cartwright, Susan Cutter, Carol Dowell, Lee Ann Grote, Travis Heskett, Jason Kelly, Carl Swinford, and Sally Swinford for their assistance with this project.
References - refereed
1. Bertschinger HU, Fairbrother JM. Escherichia coli infections. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press. 1999;431-468.
2. Amezcua R, Friendship RM, Dewey CE, Gyles C, Fairbrother JM. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E coli serogroups involved, and their antimicrobial resistance patterns. Can J Vet Res. 2002;66:73-78.
3. Nagy B, Fekete PZ. Enterotoxigenic Escherichia coli (ETEC) in farm animals. Vet Res. 1999;30:259-284.
4. Mackinnon JD. Enteritis in the young pig caused by Escherichia coli. Pig J. 1998;41:227-255.
6. Alvarez RM, Amass SF, Stevenson GW, Spicer PM, Anderson C, Ragland D, Grote L, Dowell C, Clark LK. Evaluation of biosecurity protocols to prevent mechanical transmission of transmissible gastroenteritis virus of swine by pork production unit personnel. Pig J. 2001;48:22-33.
7. Kudva IT, Blanch K, Hovde CJ. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl Environ Microbiol. 1998;64:3166-3174.
8. Sarmiento JI, Dean EA, Moon HW. Effects of weaning on diarrhea caused by enterotoxigenic Escherichia coli in three-week-old pigs. Am J Vet Res. 1988;49:2030-2033.
10. Amass SF, Stevenson GW, Anderson C, Grote LA, Dowell C, Vyverberg B, Kanitz C, Ragland D. Investigation of people as mechanical vectors for porcine reproductive and respiratory syndrome virus. Swine Health Prod. 2000;8:161-166.
11. Otake S, Dee SA, Rossow KD, Deen J, Joo HS, Molitor TW, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by fomites (boots and coveralls). J Swine Health Prod. 2002;10:59-65.
References - non refereed
5. Chase CCL. Escherichia coli in post-weaning diarrheas: Diagnosis and control. Proc Swine Dis Conf Swine Pract. Ames, Iowa: Iowa State University. 2000:15-18.
9. Bosworth BT, Casey TA. Identification of toxin and pilus genes in porcine Escherichia coli using polymerase chain reaction (PCR) with multiple primer pairs [abstract]. Gen Meet Amer Soc Microbiol. 1997;116. Abstract B-509.
