Brief communication |
Peer reviewed |
Evaluation of the use of a subunit classical swine fever marker vaccine under field conditions in Mexico
Marc Martens, DMV; Carlos Rosales, MS; Antonio Morilla, PhD
MM: Intervet International, PO Box 31 5830AA, Boxmeer, Netherlands; CR: Depto Medicina Preventiva, FMVZ, UNAM. Cd Universitaria, Mexico, DF; AM: CENID-Microbiologia, INIFAP, SAGAR; Km 15.5, carr Mex-Toluca, Palo Alto Mexico DF.
Martens M, Rosales C, Morilla A. Evaluation of the use of a subunit classical swine fever marker vaccine under field conditions in Mexico. J Swine Health Prod. 2003;11(2):81-85. Also available as a PDF
Summary
Vaccination with an E2 subunit classical swine fever (CSF) marker vaccine (Porcilis pesti; Intervet International, Boxmeer, Netherlands) appeared to limit the number of new outbreaks in an endemic area. An ELISA that identifies antibodies to the CSF Erns protein differentiated vaccinated and infected pigs in vaccinated herds.
Keywords: swine, classical swine fever, marker vaccine, eradication
Received: August 9, 2001
Accepted: February, 25, 2002
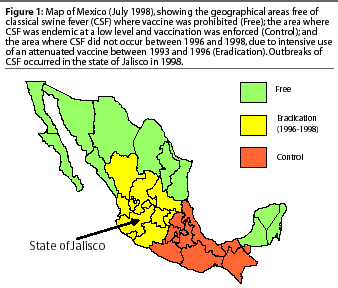
Classical swine fever (CSF) continues to be one of the most economically important diseases faced by the swine industry worldwide. In Mexico, its control has been particularly difficult in some regions because of the prevalence of small, "backyard" swine farms in which swill feeding is very common, pigs are either not vaccinated or are irregularly vaccinated, and veterinary surveillance is uncommon.1 Part of Mexico is free of CSF, including the states of Sonora in the north and Yucatán in the south-east (Figure 1). In the rest of the country, CSF is endemic, with most outbreaks occurring in "backyard pigs."1 The outlook for better control of CSF in Mexico, and perhaps eventual eradication, was enhanced by the relatively recent development of a "marker" vaccine and a complementary differential diagnostic test.2,3 The vaccine (Porcilis Pesti; Intervet International, Boxmeer, Netherlands) was first registered for use in Mexico in 1998. When used in conjunction with its complementary test, it allows for the identification of swine exposed to field strains of CSF virus (CSFV) regardless of their vaccination history. Consequently, it circumvents one of the major obstacles to the inclusion of vaccines in control and eradication programs, namely, the inability to recognize potential carriers of virulent field virus among vaccinated pigs.
The purpose of this report is to describe some of our experiences in Mexico with the use of Porcilis Pesti and a complementary differential diagnostic test.
Current CSF situation in Mexico
Presently, Mexico is divided into two geographical areas on the basis of CSF status. The CSF-free area (Free) includes eight contiguous states in the north and three contiguous states in the southeast where vaccination is prohibited (Figure 1). In the Control area, CSF is endemic at a very low level and vaccination, using conventional, attenuated CSFV vaccine, is enforced. Between 1996 and 1998, there was also an Eradication area where vaccination was prohibited after CSF disappeared as the result of an intensive program of vaccination between 1993 and 1996. Several epidemics of CSF occurred in the Eradication area during 1998, and were presumed to have been initiated when pigs carrying CSFV were introduced from the Control area.4,5 As vaccination with attenuated CSF vaccine was prohibited, animal health authorities decided to utilize a marker vaccine(Porcilis pesti) which did not contain live virus and allowed differentiation of vaccinated and CSF-infected pigs.
Outbreak of CSF in Jalisco
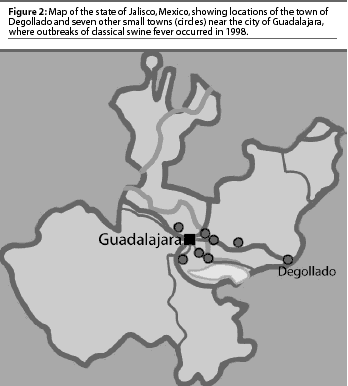
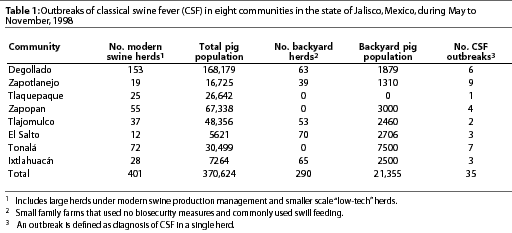
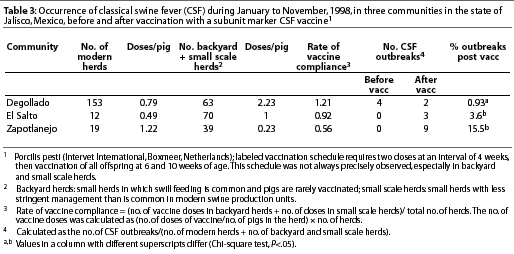
In 1998, CSF was diagnosed in the Eradication area in the state of Jalisco (Figure 2), in eight communities with a total swine population of approximately 370,000 pigs (Table 1). The first cases appeared in and around Degollado (Figure 2), a town near the city of Guadalajara with a large backyard-pig population (Table 2). Diagnosis was confirmed by serology and virus isolation.6,7 Swine producers, who knew of the availability of a marker vaccine, asked regulatory authorities to consider vaccination as a means of controlling the disease. Largely as a result of their influence, use of Porcilis Pesti was approved. Before vaccination was initiated, there were four CSF-infected farms (outbreaks) in Degollado. In the context of this report, an outbreak is defined by the diagnosis of CSF in one or more animals in a single herd.
The CSF marker vaccine and its complementary diagnostic test
The marker vaccine used in this study (Porcilis Pesti) was developed by genetic manipulation, by insertion of the gene coding for the E2 structural protein of CSFV into a baculovirus vector.8 The vaccine, which is produced commercially by replicating the genetically altered vector in cell culture, contains only the E2 protein. The E2 protein elicits CSFV neutralizing antibodies (and protective immunity) in vaccinated pigs without inducing antibodies to other CSFV proteins. The complementary diagnostic test used in this study was an ELISA using as antigen the Erns protein of CSFV, which is not present in the marker vaccine. With this Erns ELISA it was possible to distinguish pigs with different histories in regard to vaccination and exposure to field virus. The E2 ELISA was used to monitor the antibodies induced by the marker vaccine.2,3,9
Vaccination schedule and calculation of rate of vaccine compliance
The recommended vaccination schedule was two doses at a 4-week interval, administered simultaneously to all animals 4 weeks of age and older within individual herds. Thereafter, all piglets of vaccinated dams were to be vaccinated at 6 and 10 weeks of age. However, this schedule was not followed consistently. In some herds, not all pigs were vaccinated twice, and in other herds, some pigs were not vaccinated at all.
To take into consideration the number of doses used per animal in the backyard herds and larger but small scale herds, the rate of vaccine compliance for a specific region was calculated. The number of doses used in backyard herds was calculated as follows: (number of doses of vaccine/number of pigs) x number of backyard herds. The number of doses used in small scale herds was calculated similarly. The rate of vaccine compliance was calculated as follows: (number of doses in backyard herds + number of doses in small scale herds)/ total number of herds.
Testing procedures
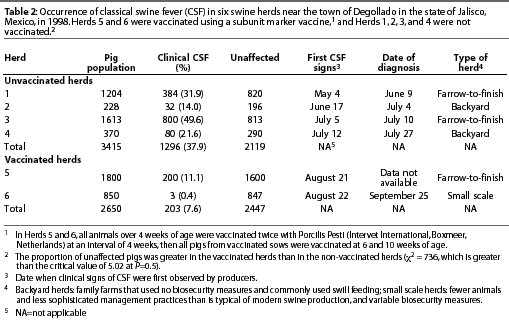
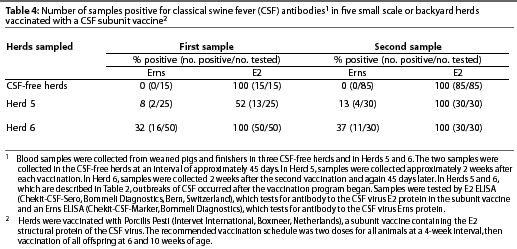
Intensive serological testing, using two different ELISA tests, was performed in three CSF-free herds and in Herds 5 and 6 (described in Table 2). In Herd 5, samples were collected approximately 2 weeks after each vaccination. In Herd 6, samples were collected approximately 2 weeks after the second vaccination and again 45 days later. In each herd, blood samples were collected from weaned and finisher pigs and tested with the E2 ELISA (Chekit-CSF-Sero, Bommeli Diagnostics, Bern, Switzerland) and the Erns ELISA (Chekit-CSF-Marker, Bommeli Diagnostics).
Statistical analysis
The numbers of healthy pigs and those showing clinical signs of CSF were compared in vaccinated and non-vaccinated herds, using a Chi-square test with a level of significance of P=.05.
The percentage of outbreaks after vaccination and the calculated rate of vaccine compliance were compared in three regions (Degollado, El Salto, and Zapotlanejo), using a Chi-square test with a level of significance of P=.05.
The percentage of outbreaks (y) in each of three regions (Degollado, El Salto, and Zapotlanejo) was regressed on rate of vaccine compliance for those regions (x) to determine the relationship between these two variables using non-linear, curve-fitting least square regression without an intercept.
Results of vaccination and testing
In Degollado, where there were 63 backyard herds and 153 larger herds with more sophisticated management, use of the vaccine appeared to have affected the occurrence of outbreaks. The prevalence of clinical signs of CSF in two vaccinated herds (7.6%) was markedly less (P<.05) than in four non-vaccinated herds (37.9%) that had been selected for comparison (Table 2; c2 = 736). The region with the greatest vaccine compliance (x) had the lowest number of outbreaks (y) of CSF (Table 3). The relationship for the three observations was described by
y=2.1342 x-3.5625 (P =.047)
In the five serologically tested herds in Degollado, the Erns test was negative and the E2 test was positive in all samples from vaccinated herds that were not infected with CSFV. In vaccinated herds that were infected, the Erns test was positive in some samples and the E2 test was positive in all samples (Table 4).
Discussion
These data show that it is possible to discriminate between vaccinated (non-infected) pigs and non-vaccinated (infected) pigs, that CSF-infected animals can be traced in a vaccinated herd by means of the Erns test,6 and that the spread of CSFV may be restricted in an area by use of a marker sub-unit vaccine. In the time that restriction of rapid virus spread saves, diagnosis can be made at other farms in a region by means of antigen and antibody ELISA tests (Erns, E2, and Erns antigen),6 so that appropriate steps may be taken to further limit outbreaks. Vaccinated pigs can always be differentiated from pigs infected with field virus.
A CSF eradication program should, of course, also include measures other than vaccination, including transport limitations, stringent biosecurity, and appropriate depopulation procedures. This study shows that use of the CSF marker vaccine in an infected area may significantly reduce clinical signs of CSF and limit the occurrence of new outbreaks. Concurrent use of the discriminatory laboratory test makes it possible to trace CSF-infected pigs in vaccinated populations. The marker vaccine may provide a valuable tool in CSF eradication programs. However, similar large-scale field-trial studies are necessary to confirm these first promising results.
Implications
- Pigs infected with classical swine fever (CSF) virus in a herd vaccinated with a subunit marker vaccine can be differentiated from non-infected, vaccinated pigs by means of the Erns ELISA.
- Use of a subunit CSF vaccine in an endemic area may limit the number of new outbreaks and thus may provide a valuable tool in an eradication program.
Acknowledgment
The authors would like to thank Theo Schetters for advice in preparing the manuscript.
References - refereed
1. Morilla Gonzales A. El control de la Fiebre Porcina Clásica por medio de la vacunación. In: Morilla A, ed. La Fiebre Porcina Clásica en las Américas. Puebla AC, Mexico: Instituto Nacional de Investigaciones Forestales y Agricolas y Pecuarias, Inter American Institute for Cooperation on Agriculture, Fundacion Produce; 2000:289-300.
2. Moorman RJ, Bouma A, Kramps JA, Terpstra C, De Smit HJ. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet Microbiol. 2000;73:209-219.
4. Morilla A, Rosales C. Reemergence of classical swine fever virus in Mexico. In: Mortilla A, Yoon KJ, Zimmerman J, eds. Trends in Emerging Viral Swine Infections. Ames, Iowa: Iowa State Pres; 2002:149-152.
5. Rosales C, Cabrera A, Castillo MA, Salas M, Ugalde E. Analsis epidemiologico de los brotes de fiebre porcina clasica. In: Morilla A, ed. La Fiebre Porcina Clasica en las Americas. Puebla AC, Mexico: Instituto Nacional de Investigaciones Forestales y Agricolas y Pecuarias, Inter American Institute for Cooperation on Agriculture, Fundacion Produce; 2000:193-206.
8. Ahrens U, Kaden V, Drexler C, Visser N. Efficacy of the classical swine fever (CSF) marker vaccine "Porcilis Pesti" in pregnant sows. Vet Microbiol. 2000;77:83-97.
References - non refereed
3. Bruderer U, Depner KR, Plagemann R, Bogner K, Keller B, Bottcher J, Schalch L, Bommeli W. Combined testing of serum samples for antigen and antibody yields optimal detection of Classical Swine Fever. Proc Third Sym Pestivirus Inf. Lelystad, The Netherlands. September 1996.
6. Macias M, Guerrero A, Toledo V, Ramirez R, Hernandez M, Lara J. Confirmación del diagnostico de FPC de lechones suceptibles a partir de muestras de porcinos en Degollado, Jalisco. Proc 34th Reunión Nacional de Investigación Pecuaria Querétaro. Querétaro, Qro, México. 1998:255.
7. Macias M, Guerrero A, Sanchez AM, Gonzales S, Delgadillo J. Aislamiento del virus de la fiebre porcona clasica en microplacas, evidenciado por immunoperoxydasa. Proc 34th Reunión Nacional de Investigación Pecuaria Querétaro. Querétaro, Qro, México. 1998:353-356.
9. Lutticken D, Drexler C, Visser N, Kaden V. The relevance of CSF marker vaccines for field use. Proc OIE Sym CSF. Birmingham, UK. July 1998. 1998:21.
