Original research |
Peer reviewed |
Further evaluation of a novel polymeric antimicrobial for the control of porcine postweaning colibacillosis
David J. Hampson, BVetMed, PhD, DSc, FRCPath, FRCVS; Alistair I. Murdoch, BVMS, MRCVS, MBA
DJH: School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch, WA, 6150, Australia. AIM: Chemeq Ltd, Technology Park, Bentley, WA, 6102, Australia. Corresponding author: Dr David J. Hampson, School of Veterinary and Biomedical Sciences, Murdoch University, Murdoch, WA, 6150, Australia; Tel: +61 89360 2287; Fax: +61 89310 4144; E-mail: D.Hampson@murdoch.edu.au.
Cite as: Hampson DJ, Murdoch AI. Further evaluation of a novel polymeric antimicrobial for the control of porcine postweaning colibacillosis. J Swine Health Prod. 2003;11(5):223-228. Also available as a PDF.
Summary
Objectives: To evaluate a new polymeric antimicrobial for the control of porcine postweaning colibacillosis (PWC).
Materials and methods: In an experimental infection study, three groups of 12 weaner pigs received either Chemeq polymeric antimicrobial in the food, a therapeutic dosage of apramycin orally, or no treatment. Three days after weaning, the pigs were challenged orally with cultures of [beta]-hemolytic Escherichia coli O8:K87:K88, then monitored daily and euthanized 11 days after weaning. In a field trial, 148 weaned pigs in a commercial swine herd were divided into five groups, receiving polymeric antimicrobial either in their water or food, apramycin in their water, a commercial E coli PWC bacterin, or no treatment. Postweaning performance was monitored.
Results: In the infection study, pigs receiving polymeric antimicrobial had less diarrhea than the apramycin-treated group (P <.01) but not the untreated control group, and had fewer hemolytic E coli in their large intestines than the control pigs (P <.05). In the field trial, pigs receiving polymeric antimicrobial had less diarrhea than pigs in the other groups (P <.05), and fewer were removed from the study because of severe PWC (P <.05).
Discussion: Antimicrobial resistance is increasing amongst PWC strains of E coli, and new antimicrobials and strategies are needed to maintain postweaning health and production. Chemeq polymeric antimicrobial reduced diarrhea after weaning, and was a useful adjunct to the control of PWC.
Implications: Chemeq polymeric antimicrobial has therapeutic advantage in the treatment and control of PWC.
Keywords: swine, weaning, diarrhea,
Escherichia coli, antimicrobial
Search the AASV web site for pages with similar keywords.
Received: October 24, 2002
Accepted: March 28, 2003
Management of antimicrobial drug resistance has become a major global imperative. For veterinarians, there is a requirement to reduce routine use of the antimicrobials that are currently available, and also to optimize the health of animals in the face of emergence of resistant bacterial pathogens. In the context of intensive pig production, postweaning colibacillosis (PWC) remains one of the most problematic bacterial diseases still occurring endemically in swine production units worldwide.1 Classical PWC is a common and severe diarrheal disease which occurs in the first 3 to 10 days after weaning. Diarrhea results from the action of one or more serotypes of [beta]-hemolytic enterotoxigenic Escherichia coli which proliferate in the proximal small intestine during this postweaning period.2 The E coli adhere to villous enterocytes via specific pili, and release enterotoxins which are responsible for loss of fluid and electrolytes, and hence cause the secretory diarrhea.3,4
The use of oral or systemic antibiotics, together with electrolyte replacement therapy, is the most common method used to treat PWC. Unfortunately, the strains of E coli associated with PWC are becoming increasingly resistant to a range of antimicrobials,5,6 and in response to this problem, a variety of alternative therapies are being developed. Recently, a novel polymeric antimicrobial, active ingredient poly (2-propenal, 2-propenoic acid) (Chemeq polymeric antimicrobial; Chemeq Ltd, Bentley, Western Australia), was reported to have potential for the control of PWC.7 In vitro studies have shown that it exerts biocidal activity against a range of bacteria, bacterial spores, fungi, and viruses, and, furthermore, that the large molecular weight of the polymer limits its absorption through the gut wall.8 This antimicrobial has a mode of action different from that of other antimicrobials in current use. It contains reactive aldehyde groups that act by initially adsorbing to, then denaturing, surface proteins of micro-organisms, thereby killing them in a non-selective way. Because of its non-specific mode of action, use of this antimicrobial is unlikely to contribute to further antibiotic resistance in enteric pathogens of food-producing animals or human beings. Chemeq polymeric antimicrobial becomes less active as it passes along the gastrointestinal tract, due to the formation of microbiologically inactive polymer-protein conjugates (unpublished data, V. Wycoco, 2002). Consequently, there is minimal impact on the microflora of the lower tract.
The purpose of the current work, comprising an experimental infection study and a field trial, was to further evaluate the efficacy and safety of the polymeric antimicrobialin the control of PWC. In the initial experimental infection study, efficacy was compared with the use of apramycin sulfate given as a treatment for diarrhea in weaned pigs inoculated with K88+ E coli. In the field trial, efficacy was compared with the use of apramycin sulfate and a commercial PWC E coli bacterin. Preliminary results of this study have been reported.9,10
Materials and methods
Experimental infection study
Animals, housing, and feeding. Thirty-six Large White x Landrace piglets were purchased from a commercial swine herd on the day of weaning at 21 days of age (Day 0). They were transported to Murdoch University, weighed, ear tagged, and allotted in a randomized complete block design to three treatment groups of 12, each of which comprised six males and six females penned separately. The pigs were housed in six adjacent pens in an isolation animal facility. Water and feed were available ad libitum, and each day uneaten feed was removed and fresh feed was supplied. Group food intake, which was the sum of the intakes of both pens in each group, was recorded daily. The diet was a commercial, antimicrobial-free, pelleted weaner diet (19% crude protein).
Study design. Beginning on Day 0, the food offered to the piglets of Group 1 was top-dressed with a controlled-release pellet containing 1% of Chemeq polymeric antimicrobial plus 0.1% sucrose. The amount added was such that the piglets were consuming approximately 30 mg of polymer per kg BW daily by Day 3. Daily from Day 4, apramycin sulfate (Apralan Soluble Powder; Elanco Animal Health, West Ryde, Australia) was administered to the piglets of Group 2. Each pig received 2 mL of a solution of apramycin sulfate dissolved in water, given orally by syringe once daily, for a dose of 15 mg per kg BW. The piglets of Group 3 received no antimicrobial treatments.
On Day 3, rectal swabs were taken from each pig and used to inoculate Columbia agar plates (Oxoid Ltd, Basingstoke, Hampshire, UK) containing 5% defibrinated ovine blood. The plates were incubated overnight at 37°C. After swabbing, the animals were dosed orally with 50 mL of an approximate 6-hour culture (109 colony forming units per mL) of [beta]-hemolytic E coli grown at 37°C in brain heart infusion broth. The strain had been recovered from a pig with PWC in a Western Australian swine herd, and had been identified as serotype O8:K87:K88, by the E coli Reference Laboratory, Bendigo, Australia. This strain has been used in previous experimental infection studies to reproduce PWC in weanling pigs.7 The sensitivity to antimicrobials of the inoculated strain and a subsequently recovered isolate were tested on Mueller-Hinton agar (Difco Laboratories, BD, North Ryde, NSW, Australia) by the Kirby-Bauer disc diffusion technique according to the United States National Committee for Clinical Laboratory Standards guidelines.11 The antimicrobials tested were neomycin, ampicillin, tetracycline, trimethoprim-sulfamethoxazole, tylosin, compound sulfonamides, and apramycin. Width of the zone of inhibition after overnight incubation was used to classify the organisms as susceptible, intermediate or resistant.11
Starting on Day 8, when clinical evidence of PWC was first observed in the control group, fecal swabs were collected daily from each pig and used to inoculate Columbia agar plates (Oxoid) containing 5% defibrinated ovine blood. The plates were incubated overnight at 37°C, and the extent of [beta]-hemolytic E coli growth on the plates was estimated in a semi-quantitative fashion, with a score of 1 for organisms present only in the inoculum, 2 for organisms also present in the first streak, 3 for organisms also present in the second streak, and 4 for organisms also present in the third streak and beyond. Group mean fecal [beta]-hemolytic E coli scores were calculated for each group from Day 8 until the end of the experiment.
A visual assessment was made of the consistency of the fecal output in a semi-quantitative manner on a daily basis, with a score of 0 for normal fecal consistency, 1 for soft fecal consistency, and 2 for diarrhea. A mean fecal consistency score was calculated for each group by totaling the fecal scores and dividing by the number of samples for the group. Within each group, the number of days that pigs had a fecal score of 1 or 2 was also calculated (diarrhea days). Mean days of diarrhea for each group were calculated over the period Day 8 to 11.
On Day 11, the pigs were weighed and then euthanized with an intravenous injection of sodium barbiturate. All pigs were subjected to postmortem examination. The gastrointestinal tract was dissected free from the mesentery. The length of the small intestine was recorded, and then it was opened at points 25% (proximal), 50% (mid) and 75% (distal) along its length. The intestinal mucosa in each area was swabbed without rinsing or scraping, and the swabs were used to inoculate Columbia blood agar plates, which were then incubated as described for fecal swabs. The cecum and the proximal colon were also opened, and the mucosa was swabbed and the swabs cultured as described. Mean [beta]-hemolytic E coli scores for the five intestinal sites were calculated as for fecal swabs.
Statistical analysis. Statistical analysis was undertaken using STATISTIX, version 7 (Analytical Software, Tallahassee, Florida). The weights of the pigs in the three groups were compared by analysis of variance (ANOVA) at the beginning and end of the trial. Fecal consistency scores, mean fecal [beta]-hemolytic E coli scores, and mean [beta]-hemolytic E coli scores at the five intestinal sites at necropsy were compared between the treatment groups of pigs using the Kruskal-Wallis ANOVA.
Field trial
A field trial was carried out in a 400-sow herd that had a history of high morbidity and mortality due to PWC associated with [beta]-hemolytic E coli O149:K91:K88. Postweaning colibacillosis was routinely managed by the use of a commercial E coli bacterin given to the piglets 7 to 10 days prior to weaning, and treatment of affected pigs with oral antibiotic preparations and injections. The antibiotic preparation was selected according to clinical response, but apramycin sulfate was considered the drug of choice. A total of 148 Large White x Landrace pigs weaned at approximately 21 days of age were randomly assigned to five treatment groups of 28 to 31 pigs, with an even distribution of male and female pigs amongst the groups. Each treatment group was housed in a single pen. Individual pigs were identified with an ear tag, and were weighed on the day of weaning (Day 0), and on Days 7 and 14. The diet was a commercial, antimicrobial-free wheat-based weaner diet containing 20% crude protein. Uneaten feed was removed daily, weighed, and replaced with fresh feed.
Pigs in Group 1 (n = 30) received antimicrobial polymer, 30 mg per kg BW daily, added manually to their drinking water in a calibrated static drinker so that intake could be monitored. Pigs in Group 2 (n = 29) received antimicrobial polymer, 30 mg per kg BW daily, top-dressed on their feed in controlled-release pellets containing 1% polymeric antimicrobial plus 0.1% sucrose. Pigs in Group 3 (n = 28) received apramycin sulfate, 12.5 mg per kg BW daily, added to their drinking water in a calibrated static drinker. Pigs in Group 4 (n = 30) were vaccinated with a commercial E coli bacterin (Weanavac; Intervet, Bendigo, Australia) administered intramuscularly to each piglet 1 week prior to weaning; Group 4 received no antimicrobial treatment. Pigs in Group 5 (n = 31) received no treatment. Staff caring for the pigs were blinded to the nature of the treatments being administered, ie, they were unaware of the types of agents being administered.
Medication for pigs in Groups 1 to 3 commenced on Day 0. The presence of diarrhea and mortality in all groups was assessed daily. On Days 4 through 7, fecal swabs were taken from all pigs with diarrhea, and used to inoculate Columbia agar plates containing 5% defibrinated ovine blood. Plates were incubated overnight at 37°C. The growth of [beta]-hemolytic E coli on the plates was estimated in a semi-quantitative fashion, using the same criteria as in the experimental infection study. Six [beta]-hemolytic E coli colonies from different pigs were submitted to the E coli Reference Laboratory, Bendigo, Australia, for serotyping, and were also subjected to antimicrobial sensitivity testing as previously described. A daily semi-quantitative assessment was made of fecal consistency, with scoring as in the experimental infection study. Feed consumption was recorded for all groups. Pigs that became moribund due to dehydration and toxemia associated with acute PWC were removed from the study and euthanized.
Statistical analysis. Statistical analysis was undertaken using STATISTIX, version 7 (Analytical Software). Mortality rates for all groups were compared using a c2 test, with rates between groups compared using two-tailed Fisher's exact tests. Group weights at the beginning of the trial were compared by ANOVA. As mean weight at the beginning of the trial was significantly different for some groups, final weights were not compared. Instead, the mean percentage change in body weight between Day 0 and 14 was calculated for each group, and mean gains were compared between groups by ANOVA. Pigs that were removed from the trial were not included in the analysis. Numbers of pigs that had diarrhea in each group were compared using ANOVA. A Tukey-Kramer multiple comparison of means test was performed when the ANOVA yielded significant results.
Results
Experimental infection study
All pigs remained healthy prior to experimental challenge, and hemolytic E coli was not detected in their feces at the time of challenge. One pig in the negative control group developed severe septic arthritis and was euthanized on humane grounds on Day 6. Pigs in all three groups gained weight during the trial, and there were no significant differences among groups in weights at the beginning or end of the trial. No mortalities occurred.
Clinical evidence of PWC was first observed on Day 8. Mean fecal consistency scores for the groups from Day 8 to 11 are recorded in Table 1. The mean fecal consistency score was lower (P <.05) for pigs receiving the polymeric antimicrobial (Group 1) than for pigs receiving apramycin (Group 2). Consistent with this, the pigs of Group 1 had fewer days of diarrhea (P <.05) compared to the pigs of Group 2 (Table 1).
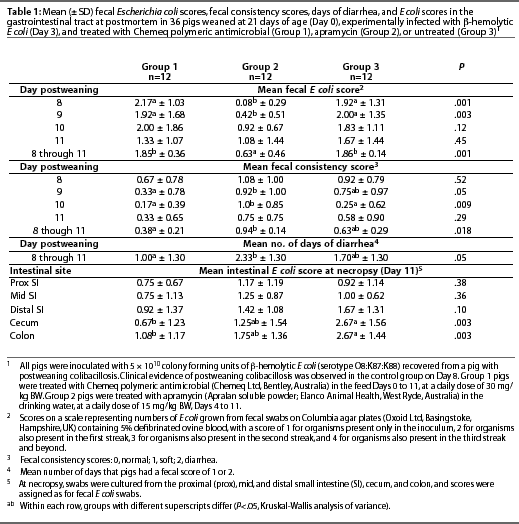
Between Days 8 and 10, [beta]-hemolytic E coli
was detected in the feces of all pigs except three from Group 2
(apramycin-treated pigs). The fecal E coli score was
lower (P <.05) for Group 2 than for the
other groups on Days 8 and 9 (Table 1). At necropsy, some fluid distension was
observed in the small intestines of all pigs, but no other gross abnormalities were
detected. Mean scores for [beta]-hemolytic E
coli throughout the small intestine were numerically lower (but not statistically
different) for the pigs treated with Chemeq
polymeric antimicrobial (Group 1) compared to both other groups
(Table 1). Mean scores for [beta]-hemolytic E
coli in the cecum and colon were lower
(P <.05) for the Group 1 pigs than for
the untreated controls (Group 3) (Table 1). The
O8:K87:K88 E coli strain used in the experiment, and a recovered isolate
which had the same serotype, were both susceptible to apramycin, but resistant to
the other antimicrobials tested.
Field trial
Results are summarized in Table 2. Pigs receiving the polymeric antimicrobial in either drinking water (Group 1) or feed (Group 2) remained healthy throughout the trial, and no mortalities occurred. Mortalities occurred in each of the other three groups, with more mortalities in those receiving apramycin (Group 3) and no treatment (Group 5) than in the other three groups. Pigs in Groups 3, 4 (vaccinated), and 5 had more diarrhea days (P<.05) and higher fecal consistency scores (P<.05) than pigs in Groups 1 and 2.
Mean weight at the beginning of the trial was less for Group 3 than for Groups 2 and 4 (P<.05). Mean percentage gain was less for pigs of Group 2 compared to pigs of Group 3, whilst no other differences were significant (Table 2).
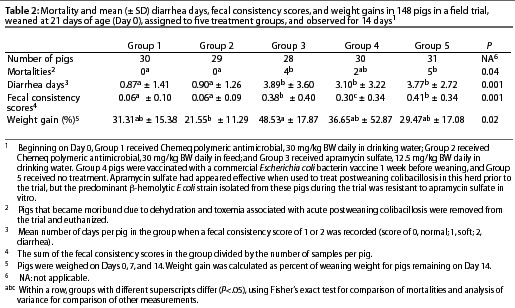
The representative [beta]-hemolytic E coli colonies from pigs with diarrhea were typed as O149:K91:K88, the same as the predominant serotype previously associated with PWC in the herd. The isolates were resistant to apramycin, compound sulfonamides, and tetracycline, and sensitive to the other antimicrobials tested. Beta-hemolytic E coli was recovered from five pigs in Group 3, two pigs in Group 4, and four pigs in Group 5, but not from pigs in either Group 1 or 2.
Discussion
The initial experimental infection study successfully reproduced PWC, although the disease was relatively mild. Untreated control pigs had a mean of only 1.7 days of diarrhea, and a mean fecal E coli score of 1.86 on Days 8 through 11, when diarrhea occurred. In some experimental studies it has been difficult to reproduce PWC,12 whilst in other studies using a similar inoculum to that used here, pigs have died with acute signs of disease within 36 hours of inoculation.7 The strain of E coli used in this study was able both to colonize the intestinal tract and induce diarrhea. Fecal shedding of hemolytic E coli was detected in all pigs except three in Group 2, the group treated with apramycin. No attempt was made to assess the K88 (F4) receptor status of the pigs, and even though the pigs were randomly assigned to their groups, it is possible that differences in receptor status between groups might have influenced the degree of E coli colonization of the small intestine, which was not detected by recording fecal shedding.
The polymeric antimicrobial was given in the food daily beginning Day 0, whilst apramycin sulfate was given daily as a post-exposure treatment starting on Day 4. The trial was designed in this way because it was likely to be several days before the pigs consumed enough feed to receive the optimal daily dose of polymeric antimicrobial. It was envisaged that if the new antimicrobial were to be used in commercial swine herds with recurrent PWC problems, it would be provided in the feed. On the other hand, there was some concern that if the apramycin was given as an oral dose at a therapeutic concentration before the experimental infection, it might completely prevent colonization in this group.
Unexpectedly, although the E coli strain used to infect the pigs was susceptible to apramycin, treatment with apramycin was not effective in preventing diarrhea, although it did significantly reduce fecal shedding of the E coli strain. In contrast, compared to the apramycin-treated group, the pigs receiving the polymeric antimicrobial had lower mean fecal consistency scores on Days 9 and 10, and less diarrhea during Days 8 through 11, but they had significantly more fecal shedding of hemolytic E coli on Days 8 and 9. Interestingly, at necropsy, the pigs receiving the polymeric antimicrobial had numerically fewer hemolytic E coli throughout their intestinal tracts than did pigs in the other two groups, and significantly fewer in the cecum and colon than the control pigs of Group 3. It has been suggested that the polymeric antimicrobial, with functional aldehyde groups, acts mainly in the small intestine, where it becomes more active as a biocidal compound in the alkaline environment (unpublished data, V. Wycoco, 2002). As this region of optimal activity is also the site of origin of the hypersecretory diarrhea, this may explain why pigs receiving the antimicrobial had less diarrhea but still had more fecal shedding of hemolytic E coli than did pigs of the other two groups. Overall, the pigs receiving the polymeric antimicrobial tended to have less diarrhea and lower E coli scores in the intestinal tract at the end of the trial than did the pigs treated with apramycin or the untreated pigs. Therefore, this study provided initial evidence suggesting that Chemeq polymeric antimicrobial might be of use in the control of PWC.
The field study was conducted on a commercial farm where PWC was a major recurrent problem. The results obtained during the trial confirmed that [beta]-hemolytic E coli of serogroup O149 were involved in the etiology of the diarrhea present on the farm. Chemeq polymeric antimicrobial given either in the water or in the feed was effective in preventing the natural colonization by hemolytic strains of E coli that was seen in the untreated group and other treated groups of pigs. Furthermore, treatment with the polymeric antimicrobial significantly reduced diarrhea and completely prevented subsequent mortalities associated with PWC. In contrast, both apramycin treatment and the use of a bacterin appeared ineffective at controlling the PWC problem that occurred on the farm. Although apramycin was used as the drug of choice on the farm, the predominant hemolytic E coli strain circulating during the study period (O149:K99:K88) was unexpectedly found to be resistant to apramycin in vitro, possibly as a consequence of its regular use. This resistance may help to explain the lack of protection achieved with apramycin in the field trial. Apramycin resistance appears to be increasing amongst E coli strains recovered from cases of PWC.6,13 Lack of efficacy of the E coli bacterin during the trial is consistent with reports of a general lack of efficacy of autogenous and other bacterins for controlling PWC.14
Although the pigs receiving the polymeric antimicrobial in the feed gained less weight than the pigs receiving apramycin, these results were influenced by the fact that the weights of the pigs that were removed because of ill-health were not included in the final analysis, and, as there were no mortalities in the pigs receiving the polymeric antimicrobial, none were removed from the analysis.
Implications
- Under the conditions of these trials, Chemeq polymeric antimicrobial was an effective treatment for the control of PWC.
- In a pen trial, where pigs were experimentally infected with K88+ E coli, treatment with the polymeric antimicrobial was more effective at reducing diarrhea than was treatment with apramycin.
- In a large-scale field trial in a swine herd with natural PWC, treatment with the Chemeq polymeric antimicrobial prevented mortalities and resulted in significantly less diarrhea than did treatment with apramycin, vaccination with an E coli bacterin, or no treatment.
- Chemeq polymeric antimicrobial appears to be effective at reducing losses associated with PWC under field conditions.
Acknowledgements
The authors are grateful to Associate Professor Ian Robertson for assistance with the statistical analysis, and to Ms Fay Bahemia and Mr Vinnie Wycoco for technical assistance.
References - refereed
1. Hampson DJ. Postweaning Escherichia coli in pigs. In: Gyles CL, ed. Escherichia coli in Domestic Animals and Humans. 2nd ed. Wallingford, UK: CAB International; 1994:171-191.
2. Bertschinger HU, Fairbrother JM. Post weaning diarrhea and edema disease. In: Straw BE, D'Allaire S, Mengeling WI, Taylor DJ, eds. Diseases of Swine 8th ed. Oxford, UK: Blackwell Science; 1999:441-454.
3. Morris JA, Sojka WJ. Escherichia coli as a pathogen in animals. In: Sussman M, ed. The virulence of Escherichia coli. London, UK: Academic Press; 1985:47-77.
5. Barton MD. The down-side of antibiotic use in pig production: the effect on antibiotic resistance of enteric bacteria. In: Cranwell PD, ed. Manipulating Pig Production VII. Werribee, Australia: Australasian Pig Science Association; 1999:194-199.
6. Amezcua R, Friendship RM, Dewey CE, Gyles CL, Fairbrother JM. Presentation of postweaning Escherichia coli diarrhea in southern Ontario, prevalence of hemolytic E. coli serogroups involved, and their antimicrobial resistance patterns. Can J Vet Res. 2002;66:73-78.
7. Hampson DJ, Buddle R, Melrose GJH. Evaluation of a novel antimicrobial polymer for the control of porcine postweaning colibacillosis. Aust Vet J. 2000;78:117-120.
11. National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests. Approved standard M2-A3. Villanova, Pennsylvania; 1984.
12. Madec F, Bridoux N, Bounaix S, Cariolet R, Duval-iflah Y, Hampson DJ, Jestin A. Experimental models of porcine postweaning colibacillosis and their relationship to postweaning diarrhoea and digestive disorders as encountered in the field. Vet Microbiol. 2000;72:295-310.
13. Mateu E, Martin M. Antimicrobial resistance in enteric porcine Escherichia coli strains in Spain. Vet Rec. 2000;146:703-705.
References - non refereed
4. Francis DH. Enterotoxigenic Escherichia coli infections in pigs and its diagnosis. J Swine Health Prod. 2002;10:171-175.
8. Melrose GJH; IP Australia, Woden, ACT, Australia. Chemotherapeutic Compositions. Australian Patent Number 701974. 1998.
9. Hampson DJ, Murdoch AI. CHEMEQ" polymeric antimicrobial for the control of experimental postweaning colibacillosis. Proc 17th IPVS Cong. Ames, Iowa. 2002;2:103.
10. Hampson DJ, Murdoch AI. CHEMEQ" polymeric antimicrobial for the field control of post- weaning colibacillosis. Proc 17th IPVS Cong. Ames, Iowa. 2002;2:104.
14. Friendship RM, Dewy CE, Amezcua R, Chernysheva L, Gyles CL. Failure of non-antibiotic treatments for the control of postweaning E. coli diarrhea. Proc 17th IPVS Cong. Ames, Iowa. 2002:1;328.
