Diagnostic notes |
Non refereed |
Serologic basis for assessment of subclinical Salmonella infection in swine: Part 1
Isabel Turney Harris, DVM, PhD
Department of Microbiology, College of Agriculture, Iowa State University, Ames, Iowa. Address correspondence to: Dr Isabel Turney Harris, Department of Microbiology, 207 Science I, Ames, IA 50011; Tel: 515-294-7058; Fax: 515-294-6019; E-mail: iharris@iastate.edu.
Cite as: Harris IT. Serologic basis for assessment of subclinical Salmonella infection in swine: Part 1. J Swine Health Prod. 2003;11(5):247-251. Also available as a PDF.
Search the AASV web site for pages with similar keywords.
This is the first part of a two-part article. Part 2 will appear in the Diagnostic notes section of the November-December (2003) issue of the Journal of Swine Health and Production.
Salmonella serology in swine
Within the last 10 years, serology has been used to determine the prevalence of salmonellae on pig farms and has been adopted by several countries into national control programs designed to reduce occurrence of salmonellae on the farm and in pork. Serology is an attractive alternative to bacteriologic methods, which have low sensitivity and which are expensive.
Serological tests for Salmonella antibodies in swine are interpreted by associating test results with a reduction in prevalence of subclinical infection in pigs (determined by bacteriologic methods) and reduced risk of carcass contamination at slaughter, rather than with the presence or absence of disease. At its present level of sensitivity and specificity, the Salmonella ELISA functions under field conditions as a herd test, ie, the responses of individual animals are evaluated in order to make a decision on the status of the whole herd. In research situations, however, where pigs are experimentally infected with pure cultures of known serovars of Salmonella, the ELISA is useful as an individual pig test. Whether Salmonella ELISA testing should be adopted in national surveillance programs as a method of reducing foodborne disease caused by Salmonella serovars depends upon the ability of the test to detect indigenous serovars, availability and ease of testing, and cost per sample. Additional considerations include correlation of the serologic response with true subclinical infection, pig performance, and risk of carcass contamination at slaughter.
Control of subclinical salmonellosis
Clinical salmonellosis in pigs is diagnosed by observation of clinical signs, gross and microscopic lesions in affected tissues, and isolation and identification of Salmonella organisms, particularly serovars Choleraesuis, Typhimurium, and several others.1 Conversely, subclinical Salmonella infection, attributable to any of the more than 2400 Salmonella serovars that have the potential to infect pigs without causing clinical disease, is of zoonotic interest due to human food safety concerns regarding pork production.2 Identification of infection, rather than disease, is the challenge at farm level for control of Salmonella in pork. Sources of this infection range from feed, water, pigs, other animals, and insects, to transport vehicles and lairage, where exposure of pigs to contaminated environments for less than 2 hours may result in contamination of the carcass with Salmonella organisms, posing the risk of disease for humans consuming pork.3-6 Elimination of salmonellae in low prevalence situations, or reduction in higher prevalence situations, has been the focus for control of zoonotic salmonellae in swine.
An epidemic of human salmonellosis in Sweden in 1952 prompted initiation of a comprehensive program of continuous surveillance by bacteriologic culture of animals, feeds, and animal products, compulsory notification of all Salmonella isolates to the Swedish Board of Agriculture, and elimination of the source of the infection. This successful program has been in operation for more than 30 years, and the prevalence of salmonellae in the Swedish pig population is reported to be less than 0.1%.7
In response to a human outbreak of salmonellosis, Denmark initiated a program in 1993 to reduce the prevalence of salmonellae in pork.8 The program encompassed guidelines for producing and testing feedstuffs, an extensive serologic surveillance of pig production herds, control of pig transport and holding before slaughter, and bacteriologic testing of meat.9 This national program, facilitated by producer ownership of slaughter facilities and traceback to the farm of origin, is based upon categorizing herds by their Salmonella prevalence levels, which are determined by results of serologic assays. Herds with high seroprevalence are subject to additional control measures up to and including penalties assessed on the carcasses. An indirect ELISA described by Nielsen et al 10 became the basis for this monitoring program.
The Danish mix-ELISA (DME), so called because the antigen is a combination of lipopolysaccharide (LPS) extractions of Salmonella Choleraesuis (O antigens 6 and 7), and Typhimurium (O antigens 1,4, 5, and 12), is used to assay serum samples collected from live animals on the farm or from meat juice (collected when a meat sample from the carcass is frozen and thawed).11 Serologic monitoring has been an efficient and cost-effective tool. The prevalence of salmonellae in Danish pork is reported to have declined from 3.5% in 1993 to 0.7% in 2000.12,13
ELISA serological tests for detection of Salmonella antibodies in swine
In 1995, a technician from our laboratory was trained at the Danish Veterinary Laboratory, Copenhagen, Denmark, to perform the DME. We have trained personnel from state laboratories in South Dakota, Minnesota, Illinois, Nebraska, and Iowa, and from private laboratories at Boehringer Ingelheim Vetmedica, Inc (Ames, Iowa), and Novartis Animal Health (Larchwood, Iowa). Other laboratories, with the purpose of increasing the sensitivity of the test, have developed indirect ELISAs based upon the DME, using the same antigens or adding antigens from different Salmonella serogroups most frequently encountered in the area or country where the test will be used.14-18 Serogroup classification is based upon the O or somatic antigens (heat stable polysaccharides) that salmonellae possess as determined by slide agglutination testing. The Salmonella antibody detection (SalAD) ELISA has been developed in the United States.16,17 Several commercial companies offer Salmonella ELISA testing on swine sera or meat juice or have produced test kits or components for laboratory use in Canada and other countries (Diakit Salmonella Swine, Maxivet Laboratories, St Hyacinthe, Quebec, Canada;18,19 VetScreen Salmonella Covalent Mix-ELISA plates, Exiqon, Vedbaek, Denmark;20,21 Salmotype Pig, Labor Diagnostik, Leipzig, Germany;22,23 HerdChek Salmonella kit, Idexx Laboratories, Osterbybrik, Sweden;24 VetGraph antibody Detection Assay test components, Ames, Iowa; Vetsign Porcine Salmonella Antibody ELISA Kit (VP020), Guildhay Ltd, Guildford, Surrey, UK; Svanovir Salmonella-Ab ELISA Svanova (SVA), Uppsala, Sweden; and Porcine Salmonella antibody ELISA kit, Biovet, St Hyacinthe, Quebec, Canada). However, no commercial test kits or components are currently available in the United States. Many laboratories have published studies regarding the sensitivities and specificities of their tests in experimental and field conditions and in comparison to the DME, and the ability of the tests to detect the Salmonella serovars predominant in the country where the test will be used. Twelve laboratories that conduct either "in-house" or commercially available Salmonella ELISA tests participated in an international trial in which a panel of well-defined sera were assayed by each laboratory. Sera from pigs experimentally inoculated with different serovars of Salmonella or potentially cross-reacting organisms, and sera from Salmonella-free pigs, were assayed.25 All tests were indirect ELISAs using LPS antigens from serogroups B (which includes Salmonella Typhimurium) and C1 (which includes Salmonella Choleraesuis), and some included Salmonella LPS from other serogroups. For each test, the sensitivities were plotted against the specificities to create a receiver operating characteristic (ROC) curve. The area below the curve was considered to be proportional to the accuracy of the test, from 0.5 (random) to 1.0 (perfect). One laboratory had a very low ROC curve area of 0.6, while the rest ranged from 0.78 to 0.87. The authors suggested that international reference serum samples should be made available to standardize tests conducted by different laboratories.
Evaluation of the DME
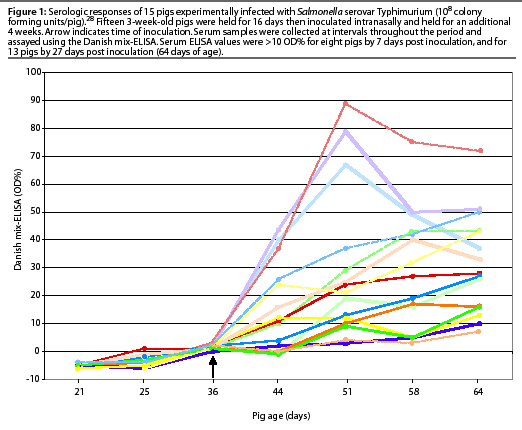
The DME can detect a serologic response in young, experimentally infected pigs less than a week after inoculation.10, 26 In a group of 37 three-month-old pigs experimentally inoculated orally with 108 colony forming units (CFU) of Salmonella Typhimurium, 86% seroconverted (optical density [OD]%>10) by day 22 post inoculation.27 The frequency of seropositive pigs peaked at 30 days post inoculation, and declined to 67% by 108 days post inoculation. There was considerable variation in the serologic response, and seroconversion was never detected in some pigs.
The variable serologic response of 15 pigs experimentally infected with Salmonella Typhimurium in another study is illustrated in Figure 1. The decline in serologic response in very young pigs within several weeks is assumed to be due to decay of passively acquired maternal antibody from the sow.28 Experimental infection with different Salmonella serovars produced varying magnitudes of detectable antibody response in individual pigs.10, 26
Nielsen et al11 evaluated the DME for use on muscle fluid (meat juice) as an alternative postmortem sample to serum. Testing of meat juice is more amenable to large scale surveillance programs, and allows for accurate identification of the serologic sample with the carcass. The agreement between results of the ELISA performed with meat juice and the ELISA performed with sera was considered acceptable, and this procedure was incorporated in the Danish Salmonella Control Program for finisher herds.
Measuring the DME serologic response
In one study of 3-week-old pigs experimentally infected with large numbers of pure Salmonella cultures and tested weekly to 108 days postinoculation, the DME response was determined by using an "experimental" or "scientific" OD% cutoff (cut point) of 10.10 This was calculated from the average OD plus eight times the SD in a group of 37 control pigs tested at the same times. Later, when the DME was evaluated for monitoring finisher age animals, the cutoff value was reassessed and set at OD% of 40 for use in the Danish surveillance program. This level was chosen so that the subsequent examination of fecal samples would be facilitated in herds in which seroprevalence was deemed moderate or high.9 In the current Danish Salmonella Control Program, the OD% cutoff has been lowered to 20;29 in this system, 10 is subtracted from the calculated OD% of the individual sample. This is called the "Salmonella Value" for the individual. The number of individuals with Salmonella Values of OD%>20 are then considered positive, and that number of animals, divided by the number tested, is the seroprevalence for the group. This number is then used to calculate the Salmonella Index, which is the basis for categorizing herds into Levels 1, 2, and 3, with 1 being the lowest seropreva-lence level. We are currently using an OD% cutoff value >=30 in the DME conducted in our laboratory, and do not subtract 10 from the individual OD% value as is done in Denmark. We determined that an OD% cutoff of 30 was optimal for epidemiologic studies in the field.30
National Salmonella surveillance programs
A national serologic surveillance program has been in place in Denmark since 1993 for breeding stock herds and since 1995 for herds producing slaughter pigs. In the Danish Salmonella Control Program for finisher herds, meat juice samples are collected monthly at slaughter and tested using the DME. The total number of samples collected from a herd yearly depends on the estimated annual number of animals sent to slaughter. From each herd sending 201 to 2000 animals per year, 60 meat juice samples are assayed with the DME each year. This sample size increases to 75 for herds sending 2001 to 5000 animals to slaughter per year and to 100 for herds marketing >5000 animals per year. The Salmonella Value for the individual sample is considered positive if the OD% is >20. Seroprevalence is determined on each slaughter group's monthly sampling and is used to calculate a "Serologic Salmonella Index," which is a weighted average of the seroprevalence for the previous 3 months, weighted 0.2, 0.2, and 0.6, least to most recent test, respectively. This index then is used to categorize the herd into one of three levels. Level 1 herds have an index of <40, Level 2 herds have an index between 40 and 70, and Level 3 herds have an index >70.29 A Level 0 category is currently being evaluated for herds in which the seroprevalence is 0 for 3 consecutive months.
Beginning in 2002, Germany initiated a voluntary Salmonella control program similar to the Danish one, and the United Kingdom introduced the Zoonoses Action Plan (ZAP) Salmonella monitoring program, also based on meat juice ELISA. The Netherlands and Belgium are considering similar programs.13 Presently, there is no national Salmonella monitoring program for pig producers in the United States or Canada. Sera collected as part of the National Animal Health Monitoring System (NAHMS) Swine 2000 Study is currently being evaluated with the DME conducted at Iowa State University, Ames, Iowa (B. Norby and E. Bush, verbal and written communication, 2002 and 2003).
ELISA sensitivity and specificity determinations
In a series of studies using pigs experimentally infected with either Salmonella Typhimurium or Infantis, the sensitivity of the DME was >95% and the specificity was 100% when compared to culture, which was used to determine the positive or negative status of the pigs.10 When the DME was conducted on meat juice, the sensitivity ranged from 81% to 89% depending upon the cutoff value used.11 In a Danish study, the sensitivity of the meat juice DME in a field situation, using an OD% cutoff of 40, was 52% for all herds, regardless of size.9
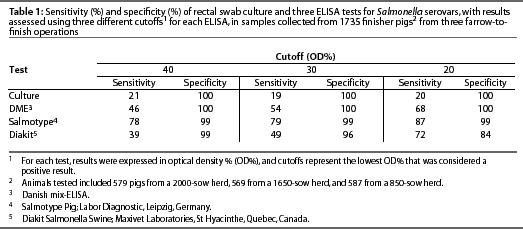
Using model-based statistical procedures not dependent upon a gold standard,31, 32 we evaluated the DME in a large cross-sectional study of three herds with varying levels of subclinical infection, comparing 1735 individual serologic responses with individual rectal swab cultures, and found the sensitivity ranged from 47% to 70%, and specificity from 75% to 66%, as the OD% cutoff was lowered from 40 to 20. The sensitivity of rectal swab culture was 20% and the specificity was 100%.30
Results obtained by testing the same set of sera with both the Salmotype22 and the Diakit18 tests were compared to results of testing the sera using the DME and culturing rectal swabs (Table 1). The sensitivity and specificity of the serologic tests varied as the cutoff changed, illustrating that optimal cutoff value depended upon the test used and the prevalence of subclinical infection, which varied among the three herds as demonstrated by culture results.30, 33 Enoe et al 34 conducted a study to estimate the sensitivity and specificity of the ELISA and culture of cecal contents and mesenteric lymph nodes, also using statistical procedures not dependent upon culture as a gold standard. The estimated ELISA sensitivity was 37% at an OD% cutoff of 40, 50% at a cutoff of 20, and 60% at a cutoff of 10, and specificity was 100%.
Correlation of serological test results with culture results
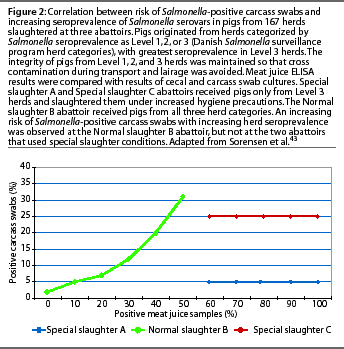
It is important to consider how serologic testing correlates to culture techniques, since culture has long been considered the gold standard for detection of Salmonella infection. Particularly, how do results of serologic tests on the serum of a live slaughter-age animal, or the meat juice from the carcass after slaughter, correlate with the presence of Salmonella organisms in the carcass? Only the organisms in the meat, not antibodies, cause disease in the consumer. Culture techniques are known to be of low sensitivity,30,34,35 and this sensitivity may vary depending upon the type of material cultured, sample size, and enrichment procedure.36-38 Culture of pooled pen feces has been shown to be useful on a herd basis 39-41 and is probably the method of choice for identifying the serovars present on a farm. Dahl 42 showed a strong correlation between serological results and culture in the individual animal, but cautioned that serologic test results could not be used for selecting individual pigs from a herd. The ELISA test was a "predictor of risk, not a statement of absolute microbiological negativity or positivity." The most conclusive evidence to date on the association of carcass culture and serological test results was an extensive study by Sorensen et al,43 involving 167 herds, comparing meat juice ELISA results at slaughter with cecal and carcass swab cultures. In this study, the integrity of pigs from each herd category (Level 1, 2, or 3) was maintained so that cross contamination during transport and lairage was avoided. Two abattoirs received pigs only from Level 3 herds and slaughtered them under increased hygiene precautions. An increasing risk of Salmonella-positive carcassswabs with increasing Salmonella seroprevalence was observed only at the abattoir that received pigs from all three herd categories of Salmonella seroprevalence (Figure 2).
References - refereed
1. Schwartz KJ. Salmonellosis. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:535-551.
2. Lax AJ, Barrow PA, Jones PW, Wallis TS. Current perspectives in salmonellosis. Brit Vet J. 1995;151:351-377.
3. Fedorka-Cray PJ, Kelley LC, Stabel TJ, Gray JT, Laufer JA. Alternate routes of invasion may affect pathogenesis of Salmonella Typhimurium in swine. Infect Immun. 1995;63:2658-2664.
4. Hurd HS, Gailey JK, McKean JD, Rostagno MH. Rapid infection in market-weight swine following exposure to a Salmonella Typhimurium-contaminated environment. Am J Vet Res. 2001;62:1194-1197.
5. Hurd HS, McKean JD, Wesley IV, Karriker LA. The effect of lairage on Salmonella isolation from market swine. J Food Prot. 2001;64:7:939-944.
6. Harris IT, Fedorka-Cray PJ, Gray JT, Thomas LA, Ferris K. Prevalence of Salmonella organisms in swine feed. JAVMA. 1997;210:382-385.
8. Wegener HC, Baggesen DL. Investigation of an outbreak of human salmonellosis caused by Salmonella enterica ssp. enterica serovar infantis by use of pulsed field gel electrophoresis. Int J Food Microbiol. 1996;32:125-131.
9. Mousing J, Thode Jensen P, Halgaard, Bager F, Feld N, Nielsen B, Nielsen JP, Bech-Nielsen S. Nation-wide Salmonella enterica surveillance and control in Danish slaughter swine herds. Prev Vet Med. 1997;29:247-261.
10. Nielsen B, Baggesen D, Bager F, Haugegaard J, Lind P. The serological response to Salmonella serovars Typhimurium and Infantis in experimentally infected pigs. The time course followed with an indirect anti-LPS ELISA and bacteriological examinations. Vet Micro. 1995;47:205-218.
11. Nielsen B, Ekeroth L, Bager F, Lind P. Use of muscle fluid as a source of antibodies for serologic detection of Salmonella infection in slaughter pig herds. J Vet Diagn Invest. 1998;10:158-163.
14. Proux K, Houdayer C, Humbert F, Cariolet R, Rose V, Eveno E, Madec F. Development of a complete ELISA using Salmonella lipopolysaccharides of various serogroups allowing to detect all infected pigs. Vet Res. 2000;31:481-490.
17. Davies PR, Morrow WEM, Jones FT, Deen J, Fedorka-Cray PJ, Gray JT. Risk of shedding Salmonella organisms by market-age hogs in a barn with open-flush gutters. JAVMA. 1997;210:386-389.
25. Van der Heijden HMJF. First International ring trial of ELISAs for Salmonella-antibody detection in swine. Berl Munch Tierarztl Wochenschr. 2001;114:389-392.
28. Baum CL. Evaluation of yogurt cultures as a method to reduce Salmonella Typhimurium in swine. [MS thesis]. Ames, Iowa: Iowa State University;2001.
29. Alban L, Stege H, Dahl J. The new classification system for slaughter-pig herds in the Danish Salmonella surveillance-and-control program. Prev Vet Med. 2002;53:133-146.
31. Enoe C, Georgiadis MP, Johnson WO. Estimation of sensitivity and specificity of diagnostic tests and disease prevalence when the true disease state is unknown. Prev Vet Med. 2000;45:61-81.
32. Hui SL, Zhou XH. Evaluation of diagnostic tests without gold standards. Stat Meth Med Res.1998;7:354-370.
36. Baggesen DL, Wegener HC, Bager F, Stege H, Christensen J. Herd prevalence of Salmonella enterica infection in Danish slaughter herds determined by microbiological testing. Prev Vet Med. 1996;26:201-213.
37. Funk JA, Davies PR, Nichols MA. The effect of fecal sample weight on detection of Salmonella enterica in swine feces. J Vet Diagn Invest. 2000;12:412-418.
38. McCall CE, Martin WT, Boring JR. Efficiency of rectal swabs and fecal specimens in detecting Salmonella carriers: correlation with numbers of salmonellas excreted. J Hyg. 1966;64:261-269.
References - non refereed
7. Wahlstrom H, Bergstrom K, Engvall A, Gunnarsson A, Lindqvist H, Berge C, Wierup M. The Swedish Salmonella control of pig and pork production. Proc IPVS. Birmingham, England. 1998;73.
12. Nielsen B, Alban L, Helle S, Sorensen LL, Mogelmose V, Bagger J, Dahl J, Baggesen DL. A new Salmonella surveillance and control programme in Danish pig herds and slaughterhouses. Proc 4th Int Sym Epidemiol Cont Salm Food Path Pork. 2001;14-21.
13. Nielsen B. Pork safety - A world overview. Proc IPVS. Ames, Iowa. 2002;121-135.
15. Widders PR, Coates KJ, Bobbitt JL, Kolega V, Pointon AM. Cultural and serological analysis of the Salmonella status of Australian pig production. Proc 2nd Int Sym Epidemiol Cont Salm Pork. 1997;221-224.
16. Gray JT, Fedorka-Cray PJ. Detection of swine exposed to Salmonella spp. Proc 3rd Int Sym Epidemiol Cont Salm Pork. 1999;46-50.
18. Letellier A, Quessy S. Determination of the Salmonella status of swine herds by the use of an ELISA test. Proc Conf Res Work Anim Dis. 2000: Abstract 42P.
19. Letellier A, Cote S, Surprenant C, Quessy S. Use of serology to evaluate the impact of clinical salmonellosis in swine on the herd status and on the contamination of pig carcasses from affected herds. Proc 4th Int Sym Epidemiol Cont Salm Food Path Pork. 2001;319-321.
20. Jauho ES, Boas U, Wiuff C, Wredstrom K, Pedersen B, Andresen LO, Heegaard PMH, Jakobsen MH. New technology for regiospecific covalent coupling of polysaccharide antigens in ELISA for serological detection. J Immunol Meth. 2000;242:133-143.
21. Wiuff C, Jauho ES, Stryhn H, Andresen LO, Thaulov K, Boas U, Jakobsen MH, Heegaard PMH. Evaluation of a novel enzyme-linked immunosorbent assay for detection of antibodies against Salmonella, employing a stable coating of lipopolysaccharide-derived antigens covalently attached to polystyrene microwells. J Vet Diagn Invest. 2000;12:130-135.
22. Gabert J, Schalch B, Greil B, Sperner B, Stolle A, Wever C, Kramer T. The use of a commercial test system (SalmotypeR-ELISA) for tracing antibodies to Salmonella in the serum of pigs. Proc 3rd Int Sym Epidemiol Cont Salm Pork. 1999;37-41.
23. Blaha T, Gaber J, Dramer T, Weber C. Testing the proficiency of the German test kit "SalmotypeR-ELISA" to identify Salmonella antibodies in porcine sera and meat juices in USA diagnostic laboratories. Proc 3rd Int Sym Epidemiol Cont Salm Pork. 1999;24-25.
24. Camitz A, Holmquist G, Ballagi A, Rodgers S. HerdChek Salmonella antibody ELISA for the serological monitoring of Salmonella infection in swine. Proc 4th Int Sym Epidemiol Cont Salm Food Path Pork. 2001;505-508.
26. Baum DH, Harris DL, Nielsen B. Serologic and bacteriologic responses of pigs infected with three serotypes of Salmonella. Proc 3rd Int Sym Epidemiol Cont Salm Pork.1999;22-23.
27. Nielsen B, Baggesen D, Bager F, Lind P. Serological diagnosis of Salmonella infections in swine by ELISA. Proc IPVS. Bangkok, Thailand. 1994.
30. Daniels MJ, Zhang Y, Erdman M, Harris IT. Estimating the accuracy of the DME in the U.S. Proc IPVS. Ames, Iowa. 2002;243.
33. Harris IT, Erdman MM, Chriswell B, Harris DL. Field determination of Salmonella exposure using serology and culture. Proc IPVS. Ames, Iowa. 2002;367.
34. Enoe C, Andersen S, Wachmann H, Sorensen LL. Estimation of sensitivity and specificity of an indirect enzyme-linked immunosorbent assay (ELISA) for detection of antibodies against Salmonella enterica in meat juice and of microbiological examination of caecal content and mesenteric caecal lymph nodes for S. enterica. Proc 4th Int Sym Epidemiol Cont Salm Food Path Pork. 2001;518-520.
35. Hurd HS, Stabel TJ, Carlson S. Sensitivity of various fecal sample collection techniques for detection of Salmonella typhimurium in finish hogs. Proc 3rd Int Sym Epidemiol Cont Salm Pork. 1999;63-64.
39. Baggesen DL, Dahl J, Wingstrand A, Nielsen B. Critical control points (CCP) in pig herds in relation to subclinical Salmonella infection. Proc IPVS. Bologna, Italy. 1996;171.
40. Erdman MM, Harris IT, Harris DL. Isolation of Salmonella using pooled pen feces from 37 U.S. swine farms. Proc 4th Int Sym Epidemiol Cont Salm Food Path Pork. 2001;547-549.
41. Bush EJ, Wagner B, Fedorka-Cray PJ. Risk factors associated with shedding of Salmonella by finishing hogs. Proc 3rd Int Sym Epidemiol Cont Salm Pork 1999;106-108.
42. Dahl J. The relation between Salmonella-shedding and the Danish Salmonella -Mix-ELISA on the pig-level. Proc 3rd Int Sym Epidemiol Cont Salm Pork. 1999;26-29.
43. Sorensen LL, Dahl J, Nielsen B. Correlation between Salmonella serology and results from bacteriological examinations of caecal contents, carcass swabs, pharyngeal swabs and caecal lymph nodes from Danish slaughter pigs. Proc 4th Int Sym Epidemiol Cont Salm Food Path Pork. 2001;316-318.
