Original research |
Peer reviewed |
Influence of age and maternal antibodies on antibody responses of neonatal piglets vaccinated against Mycoplasma hyopneumoniae
C. Hodgins, DVM, PhD; Patricia E. Shewen, DVM, MSc, PhD; Cate E. Dewey, DVM, MSc, PhD
DCH, PES: Department of Pathobiology, University of Guelph, Guelph, Ontario; CED: Department of Population Medicine, University of Guelph, Guelph, Ontari; Corresponding author: Dr D. C. Hodgins, Department of Pathobiology, University of Guelph, Guelph, Ontario, Canada N1G 2W1; Tel: 519-823-8800, ext 54758; Fax: 519-824-5930; E-mail: dhodgins@uoguelph.ca
Cite as: Hodgins DC, Shewen PE, Dewey CE. Influence of age and maternal antibodies on antibody responses of neonatal piglets vaccinated against Mycoplasma hyopneumoniae. J Swine Health Prod. 2004;12(1):10-16.
Also available as a PDF.
Summary
Objective: To assess the relative importance of age and maternal antibodies on antibody responses of neonatal piglets to a commercial Mycoplasma hyopneumoniae vaccine.
Methods: Piglets from 20 sows in each of two commercial swine operations (with serological evidence of M hyopneumoniae exposure) were vaccinated once at 2, 3, or 4 weeks of age with an M hyopneumoniae bacterin, or were nonvaccinated controls. Serum IgG antibodies were assayed by ELISA, using surface antigens of M hyopneumoniae, in serum samples collected from pigs in the first week of life and at prevaccination, 3 weeks postvaccination, and 2.5 months of age. Sows were vaccinated against M hyopneumoniae in Herd B, but not in Herd A.
Results: In Herd A, piglets had moderate titers of maternal antibodies. Vaccinated pigs had significantly higher antibody responses than nonvaccinates. Higher prevaccination titers were associated with lower responses. Age at vaccination was not associated with response to vaccination. In Herd B, piglets had high titers of maternal antibodies. Antibody titers of vaccinated pigs did not decline as rapidly as those of nonvaccinated pigs, and vaccinates had higher titers at 2.5 months of age.
Implications: Titer of maternal antibodies, but not age (ie, immaturity of immune function), is a major concern when piglets are vaccinated against M hyopneumoniae. Vaccination of pigs as young as 2 weeks of age may induce active antibody responses in the presence of moderate titers of maternal antibodies. Caution should be used in extrapolating these findings to other vaccines and vaccination protocols
Keywords: swine, Mycoplasma
hyopneumoniae, vaccination, maternal antibody,neonate
Search the AASV web site
for pages with similar keywords.
Received: November
9, 2002
Accepted: April
9, 2003
Enzootic pneumonia associated with Mycoplasma hyopneumoniae is a common and economically important disease of swine.1 Early induction of active immunity is desirable, as transmission of M hyopneumoniae may occur from sow to piglet preweaning and between piglets postweaning.1 Immune responses of neonates in general differ qualitatively and quantitatively from those of mature animals because of a combination of immaturity of immune function and suppressive effects of maternal antibodies.2,3 The age at which neonates become competent to mount immune responses to specific antigens varies with the species and the antigen of interest.3
In various species, maternal antibodies inhibit immune responses to antigens of both viral and bacterial pathogens in both replicating and nonreplicating vaccines.4,5 The effects of maternal antibodies on immune responses depend on initial serum antibody titers following absorption of colostral antibodies and on the serum half-life of these antibodies. Thus, responses to vaccination are likely to improve with age, as piglet immune function matures and titers of maternal antibodies decline. Optimal vaccination strategy must balance the advantage of delayed vaccination (ie, enhanced immune responses) with the need to induce immunity before exposure to the pathogen.
Vaccine formulation may also affect the ability of neonates to mount active immune responses in the presence of maternal antibodies. Some vaccine adjuvants6,7 moderate the suppressive effects of maternal antibodies by enhancing immunogenicity. Increasing the mass of antigen in a vaccine may also reduce the effects of maternal antibodies.8 It is thus particularly unwise to extrapolate research findings from a study using one vaccine preparation to a different population with different levels of maternal antibodies receiving a different vaccine of largely unknown formulation.
Although vaccination against M hyopneumoniae is widely practiced, many aspects of immunity to M hyopneumoniae (including the identity of protective antigens) remain unclear. This study was undertaken to assess the relative importance of age and titer of maternal antibodies on antibody responses to an M hyopneumoniae vaccine, as a starting point for more rational use of vaccines to control enzootic pneumonia. To provide a broader base for interpretation, the work was carried out in two commercial swine herds with widely differing levels of maternal antibodies.
Materials and methods
Experimental animals and design
Seven piglets from each of 20 sows in each of two commercial herds (140 piglets per herd) were selected by systematic random allocation to be vaccinated against M hyopneumoniae at 2, 3, or 4 weeks of age or to be nonvaccinated control animals. Vaccination against M hyopneumoniae was not practiced in Herd A. In Herd B, gilts were vaccinated twice before breeding with a commercial M hyopneumoniae vaccine (Suvaxyn MH/HPS; Ayerst Veterinary Laboratories, Guelph, Ontario, Canada), and received booster doses of another M hyopneumoniae vaccine (Respisure; Pfizer Animal Health, Kirkland, Quebec, Canada) 2 weeks before their estimated farrowing dates. Pigs in both herds were weaned at 14 to 19 days of age.
The youngest 20 litters of pigs born 2 to 6 days prior to the farm visit were included in the study. Litters were excluded if the pigs had been cross fostered. Weak, poor-doing pigs that were not expected to live were also excluded from the selected litters. Within selected litters, pigs were sorted by size from the largest to the smallest and were ear tagged. Pigs were systematically allocated to one of four treatment groups (vaccination at 2 weeks, 3 weeks, or 4 weeks, or nonvaccinated control) beginning with the largest pig and working in sequence towards the smallest. The fifth largest pig was allocated to the same group as the largest pig, and the sequence continued until a maximum of seven pigs had been allocated. The largest pig in the next litter was allocated to the next treatment in the sequence, and the process was continued. This procedure was followed because it was anticipated that some pigs would die before 11 weeks of age, and representation of all treatments in all litters was desired. By the end of the study, there was an average of 32 pigs per treatment group in each herd.
A single 2-mL dose of an M hyopneumoniae bacterin (Ingelvac M hyo; Boehringer Ingelheim Vetmedica, St Joseph, Missouri) was administered intramuscularly to pigs in vaccinated groups. This vaccine, consisting of killed organisms in a water-in-oil emulsion, was licensed as a one-dose vaccine for use in piglets 3 weeks of age and older; administration at 2 weeks of age therefore constituted extra-label use of this vaccine.
Blood was collected from sows in the first week after farrowing. Blood was collected from their piglets in the first week of life (Week 0, at least 24 hours after farrowing) and at the conclusion of the study (10 to 11 weeks of age). In addition, blood was collected from vaccinated pigs immediately before vaccination and 3 weeks postvaccination. Blood was collected from nonvaccinated pigs at ages corresponding to sampling ages of pigs in the three vaccination groups. A total of four blood samples were collected from vaccinated pigs and a total of eight or nine samples were collected from nonvaccinated pigs. Sera were stored at -20°C until the time of assay.
Sow sera and the first two sera collected from each piglet were tested at a 1:10 dilution for antibodies to M hyopneumoniae using the DAKO Mycoplasma hyopneumoniae ELISA Kit (DAKO, Glostrup, Denmark) to provide a means of comparing antigenic exposure in the two study herds with that in other herds of interest. This widely available commercial test uses monoclonal antibodies to a single 74-kd protein of M hyopneumoniae in a competitive ELISA to classify individuals as positive or negative for exposure to this organism, but provides only limited information as to the quantity of antibody present. In order to generate quantitative data on titers of maternal antibodies in piglets and to quantify their responses following vaccination, a modified Tween-20 ELISA was used.
Tween-20 ELISA
Serum IgG antibody titers to Tween-20 extracted antigens of M hyopneumoniae were assayed using the method of Bereiter et al,9 with modifications. Antigen was extracted from chemically inactivated culture material provided by the vaccine manufacturer (Boehringer Ingelheim Vetmedica) in order to match the antigens present in the vaccine. Immulon 2HB U-bottom 96-well microtiter plates (Dynex Technologies, Chantilly, Virginia) were coated with 100 mL per well of extracted antigen (10 mg per mL protein as determined by the Lowry10 protein assay) in carbonate coat buffer (sodium carbonate 45 mM, sodium bicarbonate 18 mM, pH 9.6), for 3 hours at 37°C. Plates were washed between steps with physiological saline containing 0.05% Tween-20 (Fisher Scientific, Fair Lawn, New Jersey). Sera were diluted in a sample buffer of 25 mM Tris buffered saline solution (pH 8.0) with 1% bovine serum albumin (ICN Biomedical, Costa Mesa, California) and 0.05% Tween-20, and dispensed to duplicate wells in 100-mL volumes. Sera were diluted as necessary in order that optical densities after color development fell within the linear response range of a dilution series of a high-titer, positive control serum, which was assayed on each plate. A gamma chain specific goat anti-swine IgG-alkaline phosphatase conjugate (Kirkegaard - Perry Laboratories, Gaithersburg, Maryland) was used with p-nitrophenyl phosphate (Kirkegaard-Perry Laboratories) as substrate for color development. Titers were calculated by the method of Sacks et al.5,11,12 Seroconversion was defined as a fourfold or greater increase in serum IgG antibody titer.
Statistical analysis
IgG serum antibody titers were transformed using a log base 2 transformation for all statistical analyses. Initially, the piglet serum IgG antibody titers were regressed on time for time points when sera were available from pigs in all groups (Week 0 and at the end of the study), controlling for litter as a random variable using the PROC MIXED procedure of SAS (SAS Inc, Cary, North Carolina). This procedure takes clustering (multiple pigs in the same litter) into account in calculating P values. When significant differences were evident among experimental groups, titers were regressed on vaccination status by comparing nonvaccinated pigs to each vaccinated group using contrasts. The interaction term herd*group was included in the model.
Analyses of antibody responses were carried out on the variable "response to vaccination," calculated as the difference between the prevaccination antibody titer and the titer in the same pig 3 weeks postvaccination. The difference was regressed on time after controlling for the random litter variable (using PROC MIXED). For each vaccination protocol (vaccination at 2, 3, or 4 weeks of age), response to vaccination was compared with changes in antibody titers in nonvaccinated control pigs during the same time interval. This was necessary for two reasons. In neonates, in the absence of antigenic stimulation, titers of maternal antibodies decline continuously over time. In addition, under field conditions, antibody responses to natural exposure to
M hyopneumoniae may occur. Comparison of changes in antibody titers in vaccinates to those in nonvaccinates over the same period and in the same environment controls for these two influences. Thus, to assess the effect of age on response to vaccination, distinct from the effects of declining titers of maternal antibodies, the prevaccination titer was included in the model as a covariate.
Results
Maternal antibodies
In Herd A, in which sows were not vaccinated against M hyopneumoniae, 25% of the sows tested positive by DAKO ELISA, and the proportion of litters with antibodies detectable by this test declined rapidly (Table 1). In contrast, in Herd B, in which all sows were vaccinated against M hyopneumoniae, all sows tested positive by DAKO ELISA, and maternal antibodies were detected in pigs in all litters up to the time of vaccination.
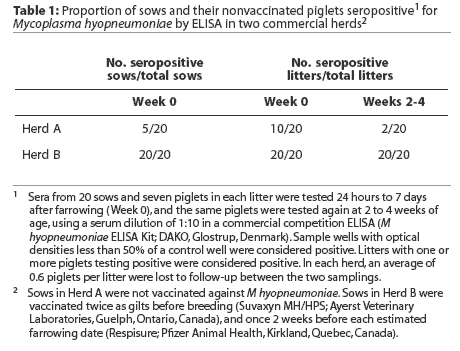
The distribution of titers of maternal antibodies in piglets under a week of age, as measured by a quantitative ELISA using Tween-20 antigen, is presented in Figure 1. Least squares means of titers were higher in piglets in Herd B (Figure 2, P < .001).
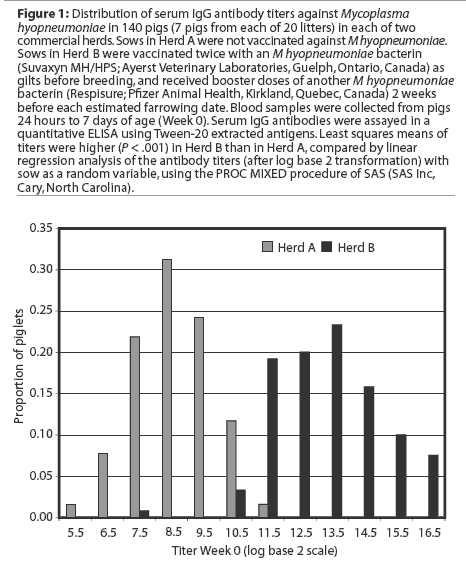
Antibody responses to vaccination
Least squares means of IgG antibody titers of pigs over the duration of the study are presented in Figure 2. Initial statistical analyses performed on combined data from the two study herds showed that serum antibody titers in the first week of life were significantly higher in Herd B than in Herd A. In addition, the interaction term herd* group was significant in models examining the effects of vaccination on antibody responses,indicating that responses differed significantly in the two herds. Therefore, subsequent analyses were performed on an individual herd basis.
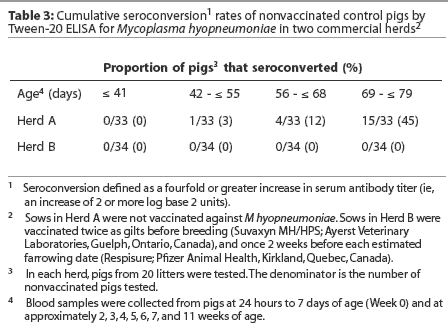
Herd A. Least squares means of maternal IgG antibody titers in the first week of life were approximately 25-fold lower in Herd A than in Herd B (Figure 2). The majority of vaccinated piglets responded to vaccination with rising IgG antibody titers (Figure 3). In pigs vaccinated at 2, 3, or 4 weeks of age, serum IgG antibody responses 3 weeks after vaccination were significantly higher than those in nonvaccinated pigs over matching time intervals (Table 2), despite a gradual increase in least squares means of antibody titers in nonvaccinated pigs after about 40 days of age in this herd (Figure 2). Indeed, 45% of nonvaccinated pigs in this herd seroconverted by 11 weeks of age (Table 3). The magnitude of antibody responses did not differ among the three vaccinated groups (with prevaccination titer as a covariate to adjust for declining titers of maternal antibodies). Higher prevaccination titers were associated with lower responses to vaccination (P < .001). At 11 weeks of age, pigs in all vaccinated groups continued to have significantly higher titers than nonvaccinates (Figure 2).
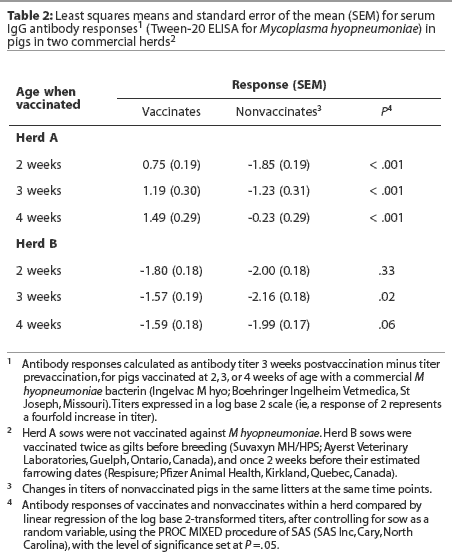
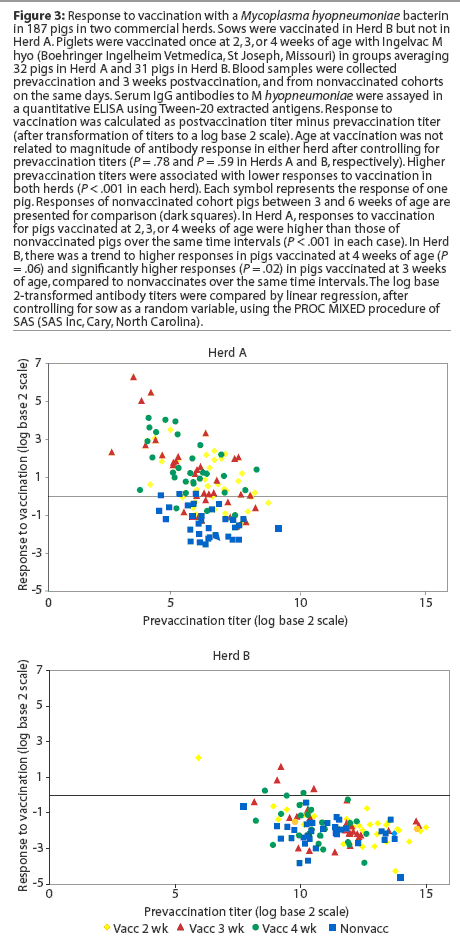
Herd B. The least squares means of maternal antibody titers were higher for pigs in Herd B than in Herd A (Figure 2), and few pigs responded to vaccination with increases in antibody titers (Figure 3). Responses following vaccination at 2 weeks of age did not differ (P = .33) from changes in titers in nonvaccinates over the matching time interval (Table 2). Responses were higher (P = .02) in pigs vaccinated at 3 weeks of age compared to nonvaccinates (Table 2). In pigs vaccinated at 4 weeks of age, there was a trend (P = .06) toward higher responses compared to nonvaccinates (Table 2). The magnitude of antibody responses among the three vaccinated groups (with prevaccination titer as a covariate to adjust for declining titers of maternal antibodies) did not differ. Higher prevaccination titers were associated with lower responses to vaccination (P < .001).
In contrast to the situation in Herd A, least squares means of antibody titers declined in all study groups up to 10 weeks of age (Figure 2), and seroconversion did not occur in any of the nonvaccinated pigs by 10 weeks of age (Table 3). In spite of the modest number of pigs with absolute increases in titers following vaccination, pigs in all vaccinated groups had significantly higher titers than nonvaccinates by 10 weeks of age. On average, the rate of decline of serum antibody titers was less precipitous for vaccinates than for nonvaccinates (Figure2).
Discussion
In nonvaccinated mature animals, positive results with the DAKO M hyopneumoniae ELISA, a qualitative commercial ELISA, suggest exposure to M hyopneumoniae. Serological results for neonatal piglets that have received colostrum provide a crude means of assessing transfer and decline of maternal antibodies. There is continuing uncertainty about which immune mechanisms are responsible for protection against M hyopneumoniae, and which mechanisms contribute to development of lung lesions. Colostral antibodies may provide partial protection against experimental challenge13 and naturally occurring disease.14 Monoclonal antibodies to an adhesin of M hyopneumoniae inhibit adherence to the cilia of tracheal epithelial cells in an in vitro model.15 Djordjevic et al,16 however, reported that serum antibody titers do not correlate with protection against experimental challenge. Thacker et al17 found that neither blood lymphocyte proliferative responses nor serum antibody titers correlated with protection on an individual pig basis, but that group antibody titers were related to group percentage pneumonic lung tissue. Recent work by Thacker et al18 suggests that interferon-[gamma]-secreting T lymphocytes and antibodies present at mucosal sites may be important in protection against experimental challenge.
For the present, until specific antigens are identified as inducing protective responses, quantitation of serum antibody responses to complex antigen mixtures serves to monitor immune responses to vaccine antigens in general, but does not predict protective effects.
Early induction of active immunity is a critical goal for vaccination programs to control enzootic pneumonia in modern pig operations. Transmission of M hyopneumoniae may occur from sow to piglets before weaning, or among piglets in the nursery.1 Vaccination is most likely to be effective if active immunity can be established before natural disease exposure. In Herd A, mean serum IgG antibody titers in nonvaccinated pigs began to rise slowly after 6 weeks of age, suggesting early exposure to M hyopneumoniae in some individuals in this herd. Indeed, 45% of nonvaccinated pigs seroconverted (fourfold or greater increases in serum antibody titers) by 11 weeks of age, indicating antigen exposure. In contrast, in Herd B, mean titers had not begun to rise by 10 weeks of age in nonvaccinates, and seroconversion was not detected. This may have been due to lack of early exposure, or high levels of maternal antibodies may have prevented effective growth of the mycoplasma. Alternatively, high titers of maternal antibodies may have interfered with detection of low titer responses.
Immunization of neonates is complicated both by immaturity of immune function and by immunosuppressive effects of maternal antibodies.2,3 Immune deficits in neonates include reduced levels of serum complement components,19 reduced expression of co-stimulatory molecules on lymphocytes,20 and reduced numbers of antigen presenting cells.21 Immune responsiveness of neonates varies with the antigen under study and the species of interest.3 In this study, antibody responses to extracted surface antigens of M hyopneumoniae did not differ significantly by age at vaccination (after adjusting for prevaccination titers of maternal antibodies). This may reflect earlier maturation of immune function in pigs in general compared to other domestic species, or may be specific for the antigens in the study vaccine. In pigs, serum levels of complement component C3 (a protein important in antigen uptake, leukocyte activation, and induction of immune memory) reach adult levels by 2 weeks of age,19 much younger than in other domestic species.22Although the effects of passive antibodies on active immune responses have been under intermittent investigation since 1892,23 there has been a recent resurgence of research interest in the immunological mechanisms involved.24-26 There is a growing consensus that although antibody (B-lymphocyte) responses of neonates are suppressed by maternal antibodies, T-lymphocyte responses are unimpaired.25,26 In addition, sensitization of B lymphocytes for anamnestic antibody responses may occur following vaccination in the presence of maternal antibodies even if no primary response is detectable.26 In Herd A, significant serum IgG antibody responses occurred in pigs vaccinated as young as 2 weeks of age in the presence of moderate titers of maternal antibodies. Mean titers rose continuously from the time of vaccination until monitoring ended at 11 weeks of age. In contrast, in Herd B, titers of maternal antibodies were approximately 25-fold higher, and mean antibody titers of vaccinates did not rise following vaccination. The rate of decline of serum antibody titers did moderate, however, compared to that of nonvaccinates, so that by 10 weeks of age, mean titers of vaccinates were significantly higher than those of nonvaccinates. This finding is consistent with a primary immune response of relatively low magnitude, and perhaps delayed kinetics, in the vaccinated pigs. Serological monitoring for a longer period of time, or experimental challenge with M hyopneumoniae, might clarify the nature of these modified responses.
The high titers of maternal antibodies present in pigs in Herd B made IgG responses difficult to detect, as IgG antibodies of the dams and offspring are not distinguished by the assay. Maternal antibody titers in the pigs were high enough that IgG derived from active responses (of the magnitude seen in Herd A) would have little impact on observed titers on a logarithmic scale. Increases in serum IgM antibodies following vaccination would provide stronger evidence of active immune responses, since titers of maternal IgM antibodies would be low by the time of vaccination because of their short half-life. Unfortunately, sera were not collected from vaccinated pigs at the 1-week interval needed for optimal detection of IgM responses.
The findings of this study have clinical implications .Morris et al27 reported that maternal antibodies to M hyopneumoniae wane at approximately 30, 45, and 63 days in pigs with low, medium, or high titers of maternal antibodies, respectively. From the results of the present study, it is evident that M hyopneumoniae vaccines may induce antibody responses before the complete disappearance of maternal antibodies. The interaction of vaccine and maternal antibodies is expected to vary with the exact formulation of the vaccine and with the titers of maternal antibodies present in the herd. Caution should be exercised in extrapolating results from this study to other vaccines and other vaccination protocols, especially in the absence of herd-specific data on levels of maternal antibodies.
To date, the specific antigens mediating protection against M hyopneumoniae have not been identified. More detailed studies of immune responses of neonatal pigs should be performed once these antigens have been characterized. Experimental challenge studies, field studies of vaccine efficacy, and mathematical models will doubtless all play their roles in defining the optimal age for vaccination to prevent enzootic pneumonia.
Implications
- Active antibody responses to M hyopneumoniae can be induced by vaccination in pigs as young as 2 weeks of age, in spite of moderate titers of maternal antibodies.
- Under the conditions of this study, maternal antibodies were associated with reduced antibody responses to vaccination.
- Age at vaccination (distinct from the effect of declining titers of maternal antibodies) was not associated with differences in magnitude of antibody responses to M hyopneumoniae.
Acknowledgements
The assistance and cooperation of the owners and managers of the commercial swine herds involved in this study are gratefully acknowledged. This study was supported financially by Ontario Pork and the Ontario Ministry of Agriculture and Food (OMAF) through the OMAF-University of Guelph agreement. The technical assistance of Dr Katherine Baldwin and Ms Jennifer Wheeler was much appreciated. Boehringer Ingelheim Vetmedica kindly supplied M hyopneumoniae antigen for assay purposes.
References
1. Ross RF. Mycoplasmal diseases. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:495-509.
2. Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331-3346.
3. Banks KL, McGuire TC. Neonatal immunology. In: Halliwell REW, Gorman NT, eds. Veterinary Clinical Immunology. 1st ed. Philadelphia: WB Saunders; 1989:193-204.
4. Siegrist CA, Córdova M, Brandt C, Barrios C, Berney M, Tougne C, Kovarik J, Lambert P. Determinants of infant responses to vaccines in presence of maternal antibodies. Vaccine. 1998;16:1409-1414.
5. Hodgins DC, Shewen PE. Serologic responses of young colostrum fed dairy calves to antigens of Pasteurella haemolytica A1. Vaccine. 1998;16:2018-2025.
6. van Binnendijk RS, Poelen MC, van Amerongen G, de Vries P, Osterhaus AD. Protective immunity in macaques vaccinated with live attenuated, recombinant, and subunit measles vaccines in the presence of passively acquired antibodies. J Infect Dis. 1997;175:524-532.
*7. deVries P, Visser IKG, Groen J, Broeders HWJ, UytdeHaag FGCM, Osterhaus ADME. Immunogenicity of measles virus ISCOMs in the presence of passively transferred MV-specific antibodies. In: Brown F, Vaccines 90. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1990;139-144.
8. Siegrist CA. Vaccination in the neonatal period and early infancy. Int Rev Immunol. 2000;19:195-219.
9. Bereiter M, Young TF, Joo HS, Ross RF. Evaluation of the ELISA and comparison to the complement fixation test and radial immunodiffusion enzyme assay for detection of antibodies against Mycoplasma hyopneumoniae in swine serum. Vet Microbiol. 1990;25:177-192.
10. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275.
11. Sacks JM, Gillette KG, Frank GH. Development and evaluation of an enzyme-linked immunosorbent assay for bovine antibody to Pasteurella haemolytica: constructing an enzyme-linked immunosorbent assay titer. Am J Vet Res. 1988;49:38-41.
12. Brogden KA, DeBey B, Audibert F, Lehmkuhl H, Chedid L. Protection of ruminants by Pasteurella haemolytica A1 capsular polysaccharide vaccines containing muramyl dipeptide analogs. Vaccine. 1995;13:1677-1684.
*13. Roof MB, Miller S, Burkhart K, Husa J. Vaccination of sows against Mycoplasma hyopneumoniae and maternal issues associated with pig vaccination [abstract]. Proc 80th Conf Res Work Anim Dis. 1999. Abstract 194.
14. Rautiainen E, Wallgren P. Aspects of the transmission of protection against Mycoplasma hyopneumoniae from sow to offspring. J Vet Med B Infect Dis Vet Public Health. 2001;48:55-65.
15. Zhang Q, Young TF, Ross RF. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect Immun. 1995;63:1013-1019.
16. Djordjevic SP, Eamens GJ, Romalis LF, Nicholls PJ, Taylor V, Chin J. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant. Aust Vet J. 1997;75:504-511.
17. Thacker EL, Thacker BJ, Boettcher TB, Jayappa H. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. Swine Health Prod. 1998;6:107-112.
18. Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am J Vet Res. 2000;61:1384-1389.
19. Tyler JW, Cullor JS, Douglas VL, Parker KM, Smith WL. Ontogeny of the third component of complement in neonatal swine. Am J Vet Res. 1989;50:1141-1144.
20. Durandy A, De Saint Basile G, Lisowska-Grospierre B, Gauchat JF, Forveille M, Kroczek RA, Bonnefoy JY, Fischer A. Undetectable CD40 ligand expression on T cells and low B cell responses to CD40 binding agonists in human newborns. J Immunol. 1995;154:1560-1568.
21. Sarzotti M, Robbins DS, Hoffman PM. Induction of protective CTL responses in newborn mice by a murine retrovirus. Science. 1996;271:1726-1728.
22. Mueller R, Boothby JT, Carroll EJ, Panico L. Changes of complement values in calves during the first month of life. Am J Vet Res. 1983;44:747-750.
23. Wiersma EJ, Coulie PG, Heyman B. Dual immunoregulatory effects of monoclonal IgG-antibodies: suppression and enhancement of the antibody response. Scand J Immunol. 1989;29:439-448.
24. Applequist SE, Dahlstrom J, Jiang N, Molina H, Heyman B. Antibody production in mice deficient for complement receptors 1 and 2 can be induced by IgG/Ag and IgE/Ag, but not IgM/Ag complexes. J Immunol. 2000;165:2398-2403.
25. Seiler P, Brundler MA, Zimmermann C, Weibel D, Bruns M, Hengartner H, Zinkernagel RM. Induction of protective cytotoxic T cell responses in the presence of high titers of virus-neutralizing antibodies: implications for passive and active immunization. J Exp Med. 1998;187:649-654.
26. Siegrist CA, Barrios C, Martinez X, Brandt C, Berney M, Cordova M, Kovarik J, Lambert PH. Influence of maternal antibodies on vaccine responses: inhibition of antibody but not T cell responses allows successful early prime-boost strategies in mice. Eur J Immunol. 1998;28:4138-4148.
27. Morris CR, Gardner IA, Hietala SK, Carpenter TE, Anderson RJ, Parker KM. Persistence of passively acquired antibodies to Mycoplasma hyopneumoniae in a swine herd. Prev Vet Med. 1994;21:29-41.
