Original research |
Peer reviewed |
Evaluation of Haemophilus parasuis control in the nursery using vaccination and controlled exposure
Simone Oliveira, DVM, MS, PhD; Carlos Pijoan, DVM, MS, PhD; Robert Morrison, DVM, PhD, MBA
SO, CP, RM: Department of Clinical and Population Sciences, College of Veterinary Medicine, University of Minnesota, St Paul, Minnesota. Corresponding author: Dr Carlos Pijoan, University of Minnesota, College of Veterinary Medicine, Department of Clinical and Population Sciences, 1988 Fitch Ave #385, St Paul, MN 55108; Tel: 612-625-2245; Fax: 612-625-1210; E-mail: pijoa001@tc.umn.edu.
Cite as: Oliveira S, Pijoan C, Morrison R. Evaluation of Haemophilus parasuis control in the nursery using vaccination and controlled exposure. J Swine Health Prod. 2004;12(3):123-128..
Also available as a PDF.
Summary
Objective: To evaluate the effectiveness of three control measures in reducing nursery mortality caused by Haemophilus parasuis, namely, a commercial vaccine, an autogenous vaccine, and controlled exposure using a low dose of live, virulent organisms.
Methods: The experiments were performed in a multi-farm system experiencing high nursery mortality (> 4.8%) due to H parasuis infection. In Experiment 1, pigs were vaccinated at weaning using a commercially available, one-dose H parasuis vaccine. In Experiment 2, pigs were vaccinated at processing and at weaning with an autogenous vaccine. In Experiment 3, pigs were orally inoculated at processing using a bacterial suspension containing a total of 105-6 colony forming units per mL of three H parasuis strains prevalent in the studied herd. Experiments 1 and 2 were repeated five times (5 consecutive weeks of production), and Experiment 3 was repeated nine times.
Results: Mortality in pigs exposed to H parasuis was lower (P < .001) than that in groups vaccinated with either commercial or autogenous vaccines.
Discussion: The lack of effect of parenteral vaccination may be due to lack of cross-protection between heterologous strains, timing of vaccination, and potential interference of maternally-derived immunity. The efficacy of controlled exposure likely reflects the induction of homologous mucosal immunity preventing pathogen systemic invasion.
Implications: The use of controlled exposure of young pigs to the prevalent H parasuis strains involved in nursery mortality provides a valuable alternative for control of H parasuis, compared to traditional vaccination using commercial or autogenous products.
Keywords: swine, Haemophilus parasuis, control, vaccination, controlled exposure
Search the AASV web site
for pages with similar keywords.
Received: July
10, 2003
Accepted: November
15, 2003
Haemophilus parasuis is normally isolated from the nasal cavity, tonsils, and trachea of healthy pigs.1,2 This organism can potentially invade the host and cause severe systemic infection characterized by fibrinous polyserositis, arthritis, and meningitis. The factors involved in systemic invasion by H parasuis have not been clearly defined.3 The potential interaction between H parasuis and some viral agents, such as porcine reproductive and respiratory syndrome virus (PRRSV),4 pseudorabies,5 and porcine circovirus type 2 (PCV2),6 has been investigated. However, no direct association between any of these agents and H parasuis has been conclusively established. The relationships between H parasuis virulence and serovar,7 genotype,8 and whole-cell protein profiles9 have also been evaluated. Although virulent strains share similar genotype and protein profiles, no specific virulence factors have been described for H parasuis.
Antibiotic treatments, or vaccination with either commercial or autogenous products, may be used to control H parasuis. The effectiveness of autogenous vaccines against homologous challenge has been demonstrated.10,11 A lack of cross-protection between different H parasuis serovars and strains has also been reported.12-14 Commercial vaccines provide satisfactory homologous protection against H parasuis strains from the same serovar group.15,16 However, vaccination failures using commercially available products have also been demonstrated, especially when the H parasuis strains prevalent in the herd differ from those included in the vaccine. In this case, autogenous vaccines may be used to reduce nursery mortality.17
Controlled exposure of 5-day-old pigs to a low dose of live, virulent H parasuis has been recently proposed as an alternative method to control nursery mortality.18 This method is based on the hypothesis that early colonization of piglets with the prevalent strains of H parasuis in the presence of maternal immunity reduces the risk of systemic infection after weaning. This control measure has been reported to reduce morbidity19 due to Streptococcus suis and mortality18 due to S suis and H parasuis systemic infection in affected nurseries. In the present study, control of H parasuis in a herd experiencing high nursery mortality was attempted using a commercial vaccine, an autogenous vaccine, or controlled exposure to live H parasuis in different cohorts.
Materials and methods
Multi-farm system
A multi-farm system (30,000 sows) experiencing high nursery mortality due to H parasuis infections 1 to 4 weeks after weaning was selected to evaluate vaccination and controlled exposure as control measures. Tissue samples collected from nursery pigs in this system were positive by polymerase chain reaction (PCR) for PRRSV, PCV2, and swine influenza virus (SIV), and were negative by PCR for Mycoplasma hyopneumoniae.
Pigs were weaned at an average of 21 days of age and transferred to off-site nurseries. Each nursery contained eight rooms, which were filled with approximately 1000 pigs each. Pigs remained in the nursery for 7 weeks.
Experimental design
The mortality data (3.25%, SD 1.1%) obtained from the last nonvaccinated group of pigs housed in the nurseries used in this study were used to calculate the sample size needed to detect a 1% decrease in mortality, with [alpha] = .05 and a power of 80%. Three experiments were performed. In Experiment 1, pigs were vaccinated with a commercial H parasuis vaccine. In Experiment 2, pigs were vaccinated with an autogenous vaccine containing two different strains. In Experiment 3, pigs were exposed to a low dose of live, virulent H parasuis (three different strains). Experiments were not concurrent, and were performed using different cohorts of pigs.
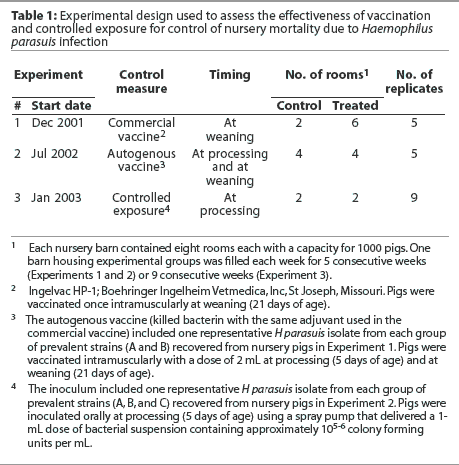
The experimental design used in each experiment is summarized in Table 1. In each experiment, a randomized complete block design was used, with barn as the blocking factor and room as the experimental unit. At the start of the trial, each barn was emptied, cleaned, and disinfected. One barn was filled each week for 5 consecutive weeks (Experiments 1 and 2) or 9 consecutive weeks (Experiment 3). Treatment was allocated to rooms within barns by randomly selecting the first room of the first barn to be used for a control or vaccinated group, and consecutive rooms were systematically allocated thereafter.
Mortality data, farm visits, and sample collecting and processing
Farm personnel were instructed to report mortality data at each nursery closeout. Mortality data used for comparison between controls and treated groups included pigs that died and pigs that were euthanized. Farm visits, for the purpose of determining the prevalent strains of H parasuis in the nursery, were completed before the beginning of Experiments 2 and 3, and during the fourth week after the first exposed pigs from Experiment 3 had entered the nursery (Table 1). Samples collected for detection of PRRSV, SIV, PCV2, and M hyopneumoniae were tested by PCR at the Veterinary Diagnostic Laboratory (University of Minnesota, St Paul, Minnesota). Samples collected for H parasuis isolation and detection by PCR were processed at the laboratory of Dr Pijoan (College of Veterinary Medicine, University of Minnesota, St Paul, Minnesota). Haemophilus parasuis fingerprinting and genetic analysis were also performed at Dr Pijoan's laboratory.
Vaccines
Pigs in Experiment 1 were vaccinated intramuscularly at weaning (21 days of age) using a single dose of a commercially available H parasuis bacterin (Ingelvac HP-1; Boehringer Ingelheim Vetmedica, Inc, St Joseph, Missouri). This vaccine is reported to protect against heterologous challenge.20
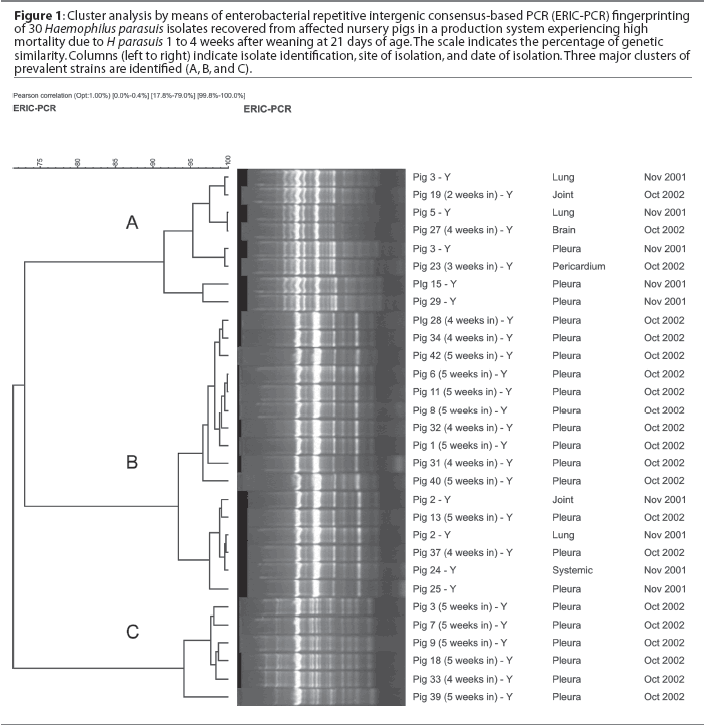
In order to select the H parasuis strains for inclusion in the autogenous vaccine (Experiment 2), 32 nursery pigs were necropsied, including 15 clinically affected pigs and 17 pigs found dead, and swabs for H parasuis isolation were collected from pleura, pericardium, peritoneum, joints, and meninges, plus lung tissue. Swabs were cultured onto blood agar with a nurse Staphylococcus aureus streak, and plates were incubated at 37°C for 24 hours. Collected swabs were also tested by PCR21 to assess the prevalence of H parasuis systemic infection in the nursery. Haemophilus parasuis isolates were genotyped by enterobacterial repetitive intergenic consensus-based PCR (ERIC-PCR) as described previously.8 Genomic fingerprints were analyzed using the BioNumerics software (Applied Maths, Kortrijk, Belgium), and one representative isolate from each prevalent group of H parasuis strains was included in the autogenous vaccine. Haemophilus parasuis isolates (one strain from genotype group A and one strain from genotype group B; Figure 1) were forwarded to Boehringer Ingelheim Vetmedica, Inc (St Joseph, Missouri), where the autogenous vaccine was produced with an adjuvant similar to that used in the commercial vaccine. Haemophilus parasuis was inactivated using 0.3% formalin. The vaccine was formulated to contain a minimum of 108 of H parasuis organisms per mL. Pigs were vaccinated intramuscularly at processing (5 days of age) and at weaning (21 days of age) using a 2-mL dose. Negative control pigs were not vaccinated.
Inoculum preparation and administration
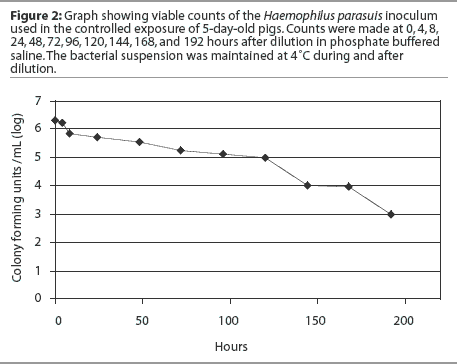
Forty-three animals were necropsied, including 41 clinically affected pigs and two pigs found dead. Pools of lung tissue obtained from these pigs were tested for PRRSV, PCV2, SIV, and M hyopneumoniae by PCR. Samples were collected and the obtained H parasuis isolates were characterized as described. One representative H parasuis isolate from each group of prevalent strains (A, B, and C) was included in the inoculum used for controlled exposure (Figure 1). Haemophilus parasuis isolates were cultured onto chocolate agar and incubated in a candle jar at 37°C for 24 hours. A seed culture containing the prevalent H parasuis strains isolated from affected animals was prepared by harvesting the bacterial growth from chocolate agar plates and suspending it in 10% sterile skim milk. The bacterial suspension was distributed in 1-mL aliquots and stored at -20°C until use. The final inoculum was prepared by diluting a 1-mL aliquot in 1 liter of sterile phosphate buffered saline (PBS).22 The inoculum was then transferred to plastic spray pumps and maintained at 4°C until use. A survivability test was performed in order to assess the viability of the inoculum after dilution in PBS. The diluted inoculum was maintained at 4°C and bacterial counts were performed at 0, 4, 8, 24, 48, 72, 96, 120, 144, 168, and 192 hours after dilution (Figure 2).
Pigs were inoculated with 105-6 colony-forming units (CFU) per mL by the oral route. At processing, each piglet in the treatment group received an oral spray (1-mL dose) of the inoculum containing 105-6 CFU per mL. Pigs in the control group were not exposed to H parasuis. The delivered amount of inoculum was validated by collecting the volume delivered by one spray in a microcentrifuge tube. All spray pumps were permanently regulated to deliver a 1-mL dose by using a pen marker. The same spray pumps were used throughout the whole experiment. Four weeks after the first group of inoculated pigs had been weaned, two or three pigs from each treated and control room were selected for euthanasia, on the basis of clinical signs characteristic of H parasuis systemic infection. Isolates from these pigs were cultured onto blood agar with a nurse streak of S aureus, and plates were incubated at 37°C for 24 hours. Swabs were tested by PCR for H parasuis. Haemophilus parasuis isolates were genotyped by ERIC-PCR and compared using the BioNumerics software.
Statistical analysis
The room (air space) was used as the experimental unit. A main effect ANOVA was performed in Statistica (StatSoft, Inc, Tulsa, Oklahoma), with percent mortality at closeout as the dependent variable and room and treatment as the independent variables. The average mortality in control and treated rooms was compared.
Results
Isolation, PCR, and genotyping of H parasuis
Haemophilus parasuis isolation and PCR results are summarized in Table 2. Based on PCR results, prevalence of H parasuis systemic infection in the nursery was 50.0% at the first visit, 53.5% at the second visit, and 35.3% at the third visit. Two prevalent genotypes (A and B) were identified among the isolates recovered from seven pigs in the first farm visit, and a third group of prevalent strains (genotype C) was identified among the isolates recovered from 20 pigs necropsied at the second farm visit. Isolates A and B were found to be still prevalent 1 year after the first isolation (Figure 1). At the third visit, when the first group of inoculated pigs had been in the nursery for 4 weeks, only strains A and B were recovered from clinically affected animals. Haemophilus parasuis was mainly isolated from nontreated control pigs (four of five isolates, compared to one isolate recovered from the joint of an inoculated pig).
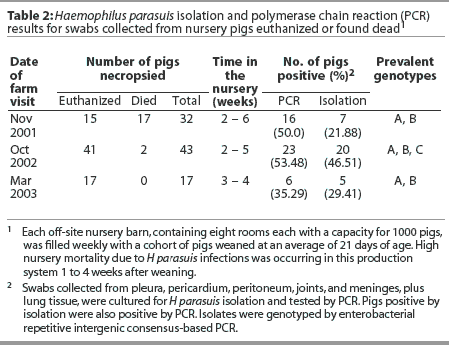
In addition to H parasuis, S suis was isolated, during the first farm visit, from the lungs of six pigs found dead and from joints of two euthanized pigs. Pasteurella multocida (20 of 43 pigs) and S suis (six of 43 pigs) were also isolated from lung samples collected during the second farm visit. Pools of lung tissue collected during the second farm visit from pigs 2 weeks postweaning were PCR-negative for PRRSV, PCV2, SIV, and M hyopneumoniae. Pools from pigs 3 and 4 weeks postweaning were PCR-positive for PRRSV and PCV2. Pools from pigs 5 weeks postweaning were PCR-positive for PRRSV, PCV2, and SIV.
Experiment 1 (commercial vaccine)
Statistical analysis showed that percent mortality was normally distributed across all rooms, with an average of 5.1% (SD 1.74%). Average mortality was 4.8% (SD 1.17%) in nonvaccinated groups (n = 10) and 5.2% (SD 1.22%) in vaccinated groups (n = 30). Room was significantly associated with mortality (data not shown), while treatment was not.
Experiment 2 (autogenous vaccine)
As in Experiment 1, percent mortality appeared to be normally distributed across all rooms, with an average of 7.4% (SD 3.28%). Average mortality was 7.7 % (SD 2.02%) in nonvaccinated groups (n = 20) and 7.1% (SD 2.47%) in vaccinated groups (n = 20). Neither room nor treatment was significantly associated with mortality.
Experiment 3 (controlled exposure)
Across all rooms, percent mortality appeared to be normally distributed, with an average of 10.4% (SD 5.99%). Average mortality was 14.3% (SD 4.98%) in control groups (n = 18) and 6.4% (SD 1.79%) in treated groups (n = 18). Treatment was significantly associated with mortality (P < .001), while room was not.
Discussion
Results of this study showed that nursery mortality was not affected by vaccination using either commercial or autogenous vaccines, while mortality was lower in pigs exposed at 5 days of age to a low dose of live, virulent H parasuis. Although these three control measures were tested in the same herd, they were used in different populations of pigs at different time points. Considering these factors, care should be taken that each study be assessed individually.
The lack of cross-protection between the commercial vaccine strain and the prevalent H parasuis strains in the herd is a potential factor that might have influenced the lack of effectiveness of the selected vaccine. Other factors to be considered include incorrect timing of vaccination, interference by maternally-derived immunity, the dose used, and possibly a need for a booster vaccination, although the manufacturer's label directions were to use only one dose.
In the second trial, the failure of vaccination using the autogenous product was unexpect-ed, since homologous protection has been reported to be effective.10,17 Previous results obtained in field trials suggest that use of autogenous vaccines may be a viable alternative to control H parasuis when commercial products are not effective.17 However, in the present study, it was demonstrated that this may not be true for all swine herds. Again, several factors might have influenced the effectiveness of the autogenous vaccine, especially timing of vaccination and interference of maternal immunity. In the present study, pigs were vaccinated with the autogenous product at processing and at weaning due to the early onset of H parasuis systemic infection in the nursery (1 to 4 weeks after weaning). When pigs are initially vaccinated at such an early age, there is always a concern regarding interference of maternal immunity in the development of the active immune response induced by the vaccine. However, some studies have demonstrated that maternal immunity may not always interfere with the immune response. Solano-Aguilar et al15 tested a commercial vaccine containing H parasuis serovars 4 and 5, using two doses, and showed that vaccinated pigs born to vaccinated gilts were protected against challenge with a virulent H parasuis strain, whereas some vaccinated pigs born to nonvaccinated gilts developed central nervous system signs and lameness. Baumann and Bilkei16 also demonstrated that vaccination of sows and their piglets resulted in protection against homologous challenge. Further studies are necessary to better characterize the potential interference of maternal antibodies in pig vaccination. Another factor that might have influenced the outcome observed in Experiment 2 was the nonrandomized distribution of experimental groups in rooms within barns. This difference may have impaired the observation of a greater effectiveness of the autogenous vaccine in the treatment group.
Another potential factor to be considered is that the autogenous vaccine was missing one of the prevalent genotypes later identified in the nursery (type C). Genotyping results showed that strains A and B, which were isolated in the previous year, were still prevalent in the herd. A third prevalent strain (C) was identified after the second sampling. This strain was not included in the autogenous vaccine. Strain C was later included in the inoculum used for controlled exposure. A general increase in mortality was observed in the second experiment compared with the first experiment. This increase in mortality coincided with a PRRS outbreak in the system. Mortality was even higher in the control group after the third experiment compared with controls in the first and second experiments. This time, SIV was identified as the main agent co-infecting nursery pigs. However, the association between PRRS or SIV and H parasuis co-infection in the nursery has not been established.
Regarding controlled exposure, Pijoan et al23 hypothesized that early mucosal colonization of piglets with the herd's prevalent strains of H parasuis while they are still protected by maternal immunity might reduce the risk of systemic infection after weaning. Following this hypothesis, it would be desirable that a large number of pigs be exposed to these potentially virulent strains before they become susceptible (ie, with waning of maternal antibody levels). Some practices currently used in modern swine production, such as early weaning and three-site production systems, may reduce exposure to these virulent strains, or spread of infection among pigs. Kirkwood et al24 demonstrated that levels of colonization by H parasuis appear to be influenced by weaning age. Their study showed that levels of colonization were lower in pigs weaned at 14 days than in pigs weaned at 28 days.
The mechanisms involved in development of protective immunity after controlled exposure to H parasuis have not been clearly defined. Nielsen25 demonstrated that exposure of specific-pathogen-free pigs to an aerosol containing live, apathogenic H parasuis strains resulted in development of circulating antibodies, and protected pigs against heterologous challenge with a virulent strain. However, further studies are necessary to better characterize the mechanisms involved in development of protective immunity by pigs exposed to live, virulent H parasuis.
In the present study, nursery mortality was significantly lower in treated groups after controlled exposure than in untreated control groups. We have previously reported that after colonization of piglets with a low dose of live, virulent H parasuis, nursery mortality was 36.6% lower in the exposed group than in the control group.18 Four factors may have improved the results of the colonization trial in the present study compared with our previous report. The sample size and number of replicates (8000 pigs per group, five to nine replicates) used in the present study were considerably larger than those in the previous report (50 pigs per group, two replicates). The herd used in the present study had a greater prevalence of systemic infection and mortality due to H parasuis. The dose of inoculum used in 5-day old pigs was higher in the present study (1 x 105-6 CFU per mL) than in the previous study (7 x 103 CFU per mL). The allocation of treated and control groups in the nursery, as well as the experimental unit used for statistical analysis, differed between studies. In the present study, treated and control groups were allocated to different rooms in a single barn, and room, or air space, was considered the experimental unit. In our previous study, all groups were allocated to different pens in the same nursery barn (ie, the same air space), and the pig was considered the experimental unit.
Results obtained in the present study demonstrated that nursery mortality may be significantly reduced after early exposure of pigs to live, virulent H parasuis, compared with the results of using autogenous and commercial vaccines. Controlled exposure has several advantages compared with traditional vaccination, including lower cost and reduction of workload. Furthermore, timing does not seem to be an issue with controlled exposure, whereas maternal immunity may interfere with pig vaccination. There are some concerns regarding the safety of this method. The interaction between PRRS virus and H parasuis has not been scientifically demonstrated. However, field experiences suggest that these organisms may co-infect nursery pigs, resulting in increased mortality compared with either pathogen alone. It would appear to be counterindicated to inoculate pigs with live, virulent H parasuis if PRRS virus infection is active in the sow farm. The vaccination results obtained in this study suggest that the use of commercial and autogenous vaccines to control H parasuis infections must be critically evaluated.
Implications
- Controlled exposure may be an alternative to other methods for control of H parasuis systemic infection in the nursery.
- Under the conditions of this study, controlled exposure was more effective than use of autogenous or commercial vaccines in reducing nursery mortality due to H parasuis systemic infection.
- Although the interaction of PRRSV and H parasuis has not been scientifically proven, as a safety measure, nursery pigs should not be exposed to live, virulent H parasuis when there is active PRRSV infection in the sow herd.
Acknowledgements
The authors would like to thank the National Pork Board for funding this project. We also would like to thank Dr Butch Baker, Ken Cantrell, and Dr John Kolb for collaborating in this project.
References
1. Møller K, Kilian M. V factor-dependent members of the family Pasteurellaceae in the porcine upper respiratory tract. J Clin Microbiol. 1990;28:2711-2716.
2. Vahle JL, Haynes JS, Andrews JJ. Experimental reproduction of Haemophilus parasuis infection in swine: clinical, bacteriologic, and morphologic findings. J Vet Diag Invest. 1997;7:476-480.
3. Nicolet J. Overview of the virulence attributes of HAP-group bacteria. Can J Vet Res. 1990;54:S12-S15.
4. Solano GI, Segales J, Collins JE, Molitor TW, Pijoan C. Porcine reproductive and respiratory syndrome virus (PRRSv) interaction with Haemophilus parasuis. Vet Microbiol. 1997;55:247-257.
5. Narita M, Kawashima K, Matsuura S, Uchimura A, Miura Y. Pneumonia in pigs infected with pseudorabies virus and Haemophilus parasuis serovar 4. J Comp Path. 1994;110:329-339.
6. Kim J, Chung H, Jung T, Cho W, Choi C, Chae C. Postweaning multisystemic wasting syndrome of pigs in Korea: prevalence, microscopic lesions and coexisting microorganisms. J Vet Med Sci. 2002;64:57-62.
7. Kielstein P, Rapp-Gabrielson VJ. Designation of 15 serovars of Haemophilus parasuis on the basis of immunodiffusion using heat-stable antigen extracts. J Clin Microbiol. 1992;30:826-865.
8. Oliveira S, Blackall PJ, Pijoan C. Characterization of the diversity of Haemophilus parasuis field isolates by serotyping and genotyping. Am J Vet Res. 2003;64:435-442.
9. Nicolet J, Paroz PH, Krawinkler M. Polyacrylamide gel electrophoresis of whole-cell proteins of porcine strains of Haemophilus. Int J Syst Bacteriol. 1980;30:69-76.
10. Smart NL, Miniats OP. Preliminary assessment of a Haemophilus parasuis bacterin for use in specific pathogen free swine. Can J Vet Res. 1989;53:390-393.
11. Miniats OP, Smart NL, Ewert E. Vaccination of gnotobiotic primary specific pathogen-free pigs against Haemophilus parasuis. Can J Vet Res. 1991;55:33-36.
12. Miniats OP, Smart NL, Rosendal S. Cross-protection among Haemophilus parasuis strains in immunized gnotobiotic pigs. Can J Vet Res. 1991;55:37-41.
13. Rapp-Gabrielson V, Kocus GJ, Clark JT, Stephen KM. Haemophilus parasuis: immunity in swine after vaccination. Vet Med. 1997;92:83-90.
14. Takahashi K, Nagai S, Yagihashi T, Ikehata T, Nakano Y, Senna K, Maruyama T, Murofushi J. A cross-protection experiment in pigs vaccinated with Haemophilus parasuis serovars 2 and 5 bacterin, and evaluation of a bivalent vaccine under laboratory and field conditions. J Vet Med Sci. 2001;63:487-491.
15. Solano-Aguilar GI, Pijoan C, Rapp-Gabrielson V, Collins J, Carvalho LF, Winkelman N. Protective role of maternal antibodies against Haemophilus parasuis infection. Am J Vet Res. 1999;60:81-87.
16. Baumann G, Bilkei G. Effect of vaccinating sows and their piglets on the development of Glässer's disease induced by a virulent strain of Haemophilus parasuis. Vet Rec. 2002;151:18-21.
17. Smart NL, Hurnik D, MacInnes JI. An investigation of enzootic Glasser's disease in a specific-pathogen-free grower-finisher facility using restriction endonuclease analysis. Can Vet J. 1993;34:487-490.
18. Oliveira S, Batista L, Torremorell M, Pijoan C. Experimental colonization of piglets and gilts with systemic strains of Haemophilus parasuis and Streptococcus suis to prevent disease. Can J Vet Res. 2001;65:161-167.
19. Torremorell M, Pijoan C, Dee S. Experimental exposure of young pigs using a pathogenic strain of Streptococcus suis serotype 2 and evaluation of this method for disease prevention. Can J Vet Res. 1999;63:269-275.
20. Ingelvac HP-1 [package insert]. St Joseph, Missouri: Boehringer Ingelheim.
21. Oliveira S, Galina L, Pijoan C. Development of a PCR test to diagnose Haemophilus parasuis infections. J Vet Diag Invest. 2001;13:495-501.
22. Morozumi T, Hiramune T. Effect of temperature on the survival of Haemophilus parasuis in physiological saline. Natl Inst Anim Health Q. (Jpn). 1982;22:90-91.
*23. Pijoan C, Torremorell M, Solano G. Colonization patterns by the bacterial flora of young pigs. Proc AASP. 1997:463-464.
24. Kirkwood RN, Rawluk SA, Cegielski AC, Otto AJ. Effect of pig age and autogenous sow vaccination on nasal mucosal colonization of pigs by Haemophilus parasuis. J Swine Health Prod. 2001;9:77-79.
25. Nielsen R. Pathogenicity and immunity studies of Haemophilus parasuis serovars. Acta Vet Scand. 1993;34:193-198.
* Non-refereed reference.
