Literature review |
Peer reviewed |
Risk factors associated with Salmonella prevalence on swine farms
Julie Funk, DVM, MS, PhD; Wondwossen Abebe Gebreyes, DVM, PhD, Diplomate ACVPM
JF: Department of Veterinary Preventive Medicine, College of Veterinary Medicine, The Ohio State University, Columbus, Ohio. WAG: Department of Population Health and Pathobiology, College of Veterinary Medicine, North Carolina State University, Raleigh, North Carolina. Corresponding author: Dr Julie Funk, Department of Veterinary Preventive Medicine, College of Veterinary Medicine, The Ohio State University, 1920 Coffey Road, Columbus, OH 43210; Tel: 614-247-6635; Fax: 614-292-4142; E-mail: funk.74@osu.edu.
Cite as: Funk J, Gebreyes WA. Risk factors associated with Salmonella prevalence on swine farms. J Swine Health Prod. 2004;12(5):246-251.
Also available as a PDF.
Summary
This article reviews on-farm risk factors that have been associated with the prevalence status of Salmonella in swine. Salmonellosis is the second most common etiological cause of bacterial human foodborne illness in the United States, and most cases can be attributed to contaminated food products. Reduction of human foodborne salmonellosis has become a public health priority both nationally and internationally. Public health concerns, increased stringency of regulatory limits at slaughter, and competition for international market share are likely to increase interest in on-farm Salmonella control.
Keywords: swine, Salmonella,
prevalence, risk factors
Search the AASV web site
for pages with similar keywords.
Received: July
15, 2003
Accepted: February
16, 2004
An estimated 1.5 million cases of nontyphoidal salmonellosis in humans occur yearly in the United States, and nearly all cases are foodborne.1 This tremendous burden on public health has made reduction of human salmonellosis a key public health objective.2 Salmonellae are ubiquitous organisms, and the vertebrate gastrointestinal tract is considered to be their biological niche. Although infection may result in clinical disease, it has long been recognized that swine may be asymptomatic carriers of salmonellae.3-12 In the United States, the number of farms positive for Salmonella has been estimated to range from 38.2 to 83%, and the number of positive pigs from 6 to 24.6%.13,14
Beyond the potential impact on domestic public health and market stability, contamination of pork products with salmonellae may put the pork export market at risk.15,16 With the coordination of "farm-to-table" Salmonella control programs by many European pork producers (among the United States' major competitors for export markets),17 demonstration of effective control measures may be important for maintaining international market share. Yet wholesale adoption of pre-existing control programs may not be practical in the United States due to differences in production systems, industry structure, and regulatory organization.
Significant strides have been made at decreasing Salmonella contamination in one link of the US pork chain, namely, at slaughter and processing. The Pathogen Reduction: Hazard Analysis and Critical Control Point (HACCP) Systems18 established performance standards at slaughter and processing plants, which has resulted in decreased contamination of product with salmonellae.19 It is expected that standards at slaughter and processing will become more stringent, creating pressure from packers and processors to reduce prevalence of Salmonella-positive swine through on-farm interventions.
The focus of this review is on the on-farm risk factors for Salmonella infection in swine, that have been identified through epidemiological investigations. Promising interventions that have thus far been described predominantly in experimental settings (vaccines, antimicrobial treatments, and competitive exclusion) are not included. Additionally, potential risks beyond the "farm gate" (eg, exposure during transport and lairage and contamination of carcasses during slaughter) are not included in this review for the main purposes of brevity and a focus on potential on-farm interventions. It is also important to recognize that most reviewed studies have been conducted in European herds, and the epidemiology of Salmonella infection, as well as the impact and feasibility of interventions, may not be directly applicable to the situation in US herds. Yet these risk factors suggest promising areas for further investigation of Salmonella control on US swine farms, and many warrant further investigation (which should include field trials) of their efficacy under US management systems.
In a 1996 review of the literature,20 "total farm hygiene" was identified as the most important risk factor for Salmonella infection. "Total farm hygiene" was defined broadly by the authors to encompass many aspects of swine production techniques that might be considered good production practices, including cleanliness, housing, biosecurity, feeding practices, water source, and antimicrobial use. For the most part, the literature since that period has continued to focus on these "hygienic" approaches to Salmonella control.
Humans as vectors
Biosecurity-related practices regarding swine farm personnel and visitors have been associated with decreased risk of infection of swine with salmonellae. Researchers have found that hand washing21 and access to toilets and hand-washing facilities22 have been associated with decreased Salmonella prevalence on swine farms. Provision of areas where clothes and footwear could be changed prior to entry into pig areas was associated with reduced Salmonella seroprevalence in Danish market swine,21 but not in Dutch herds.23 It has also been reported that in herds with relatively more humans on site daily, there was an increased risk of high fecal shedding of salmonellae,22 suggesting that increased human traffic on farms increases the pigs' risk of infection. It is unclear whether personnel hygienic practices are directly related to risk of infection of pigs with salmonellae or simply serve as a proxy measure of a pork producer's overall attitude about biosecurity. It does suggest that improved personnel hygiene may be an important intervention for infection of swine with salmonellae. The relatively small cost incurred may be offset by decreased transfer of other performance-impairing pathogens.24,25
Flooring types
The type of flooring on which pigs are reared has been evaluated in epidemiological investigations. The biological premise for its importance is that certain types of flooring decrease pig contact with fecal material, resulting in decreased fecal-oral transmission among pigs. Salmonella prevalence was lower in swine housed on fenestrated flooring (eg, concrete slats) than in pigs housed on flush-gutter flooring.13,26
Housing contamination
Contamination of the resident environment of animal housing has been implicated in many studies as a source of Salmonella infection.7,27-34 Salmonellae are capable of surviving 6 years or more in the environment,35,36 and the challenges of cleaning and disinfecting animal housing are well documented. 37-40 Concrete, a common material for swine flooring, is a difficult surface to clean and disinfect. After cleaning and disinfection of swine housing facilities, rough-surfaced concrete was more likely to have high levels of residual contamination (measured by aerobic plate counts) than smoother surfaces.39 Substandard cleaning and disinfection may allow Salmonella organisms to remain as contaminants on floors, as determined by culture.41 Surprisingly, in the Netherlands, the odds were greater for higher Salmonella seroprevalence in production systems where barns were cleaned and disinfected between groups of pigs compared to systems in which floors and buildings were cleaned but no disinfectant was used.23 The authors speculated that producers who use disinfectants clean less adequately, with the idea that remaining microbes will be dealt with by the disinfectant. Terminal disinfection, either through fogging or fine mist of formaldehyde, decreases but does not eliminate Salmonella contamination in poultry houses.38-40 Human health risks are associated with use of formaldehyde, and the benefits of its use as a disinfectant should be considered. To date, no controlled field trials have studied the impact of cleaning and disinfection practices on Salmonella prevalence in swine. Although it appears to be common sense to reduce contamination of the pig's environment, there is little indication as to what cleaning and disinfection protocols are most effective for Salmonella contamination control, let alone the economic feasibility of these interventions. There is a need to evaluate the components of cleaning and disinfection practices in use that are both effective and feasible for reduction of Salmonella contamination in swine housing.
Pig flow management
Pig flow practices that are well recognized as important for reducing production-impairing swine diseases (ie, all in-all out pig flow)42-44 are often suggested for Salmonella control, yet few studies identify the association of this practice with decreased Salmonella prevalence. The biological premise is that the combination of cleaning and disinfecting the facility between groups of pigs and segregating age groups decreases the potential for Salmonella exposure and infection. On Danish farms that used all in-all out production management and also provided areas for personnel to change clothing and boots prior to entering or leaving the pig areas, herds were nearly three times less likely to be seropositive than on farms that did not use these management practices.21 There was no reduction in risk of having a seropositive herd on farms that did not use both practices, ie, that either provided a changing area or used all in-all out flow, but not both. On the other hand, in another study of Danish swine, all in-all out pig flow was associated with increased Salmonella seroprevalence, although this result was based on a crude odds ratio, not adjusted for other management practices on the farm.45 Salmonella prevalence may be high on farms with all in-all out production. In a study of three-site production systems with all in-all out management in the United States, Salmonella prevalence in finishers ranged from 0% to more than 70%.22 The limited and contradictory evidence for all in-all out pig flow as a means of Salmonella control warrants further investigation prior to its recommendation for that specific purpose.
Importance of sow-to-pig transmission
Many investigators have reported relatively high Salmonella prevalence in breeding gilts and sows.41,46,47 Beyond the food safety risk when the sow ultimately enters the food chain, the importance of vertical transmission from the sow to her offspring has been only minimally addressed. Several authors have demonstrated that piglets may be infected early in life.6,41 Efforts to use segregated early weaning to prevent sow-to-pig transmission has had mixed results that are likely to be at best farm-specific in success.48-50 It is paradoxical that Salmonella serovars isolated from a sow often differ from those isolated from her piglets,41 which might be explained by sampling error, colostral protection, or differential infection efficiency of serotypes in pigs of different ages. Recent epidemiological surveys in Denmark51 have suggested that pigs produced from sow herds with high Salmonella seroprevalence are at greater risk for isolation of the specific serovar Salmonella Typhimurium.
More research is necessary to evaluate the importance of the sow's Salmonella infection status on the risk of infection in her offspring. If the sow herd is identified as a major source of exposure to Salmonella infection for growing pigs, this has important implications for the breadth and costs of surveillance and control programs.
Risk to swine posed by other vertebrate species
Since all vertebrates are susceptible to infection with Salmonella serovars, contact with other animal species may pose an infection risk to swine herds. The risk posed by having other domestic species on a farm with swine has been variable in the literature. Having other domestic animals on the same farm as finisher pigs has been associated with increased Salmonella prevalence.22 However, many other researchers have found no association between Salmonella infection and the presence of domestic animals other than the target species.24,40,52-54
Domestic cats residing on swine farms may shed salmonellae.55 Pests (eg, rodents, wild birds, and other wildlife species) have often been implicated as potential sources of salmonellae for swine. Several investigators have demonstrated that mice and rats on farms may be infected with salmonellae, often with the same serovars as the domestic species investigated.55-60 Many cross-sectional investigations have isolated Salmonella organisms from free-living birds at prevalence rates ranging from 0% to more than 50%.61-65 There is circumstantial evidence that sea gulls were responsible for two Salmonella outbreaks in Scotland.61,63 Wild birds near broiler houses have been found to shed salmonellae at relatively high frequencies.65 Foxes near poultry farms have been identified as shedding salmonellae.60
Economic benefits of pest control on farms, external to Salmonella control (eg, prevention of building damage and control of other diseases), may offset the costs of pest control, justifying these interventions and possibly also resulting in decreased Salmonella risk for swine.
Risks posed by invertebrate species
It has been recognized that flies55,60 and beetles60,66 (both mature and immature stages) may be vectors for Salmonella organisms. In fact, recent research suggests that the free-living nematode Caenorhabditis elegans may be persistently infected with salmonellae.67 Although no epidemiological investigations have been performed to discern the attributable risk associated with invertebrate species, it appears that they may at least serve as potential reservoirs and vectors on farms.
Risk factors associated with feed
Risk factors associated with feed may be divided into two major categories: feed as a source of salmonellae due to contamination, and the impact of feed ingredients and physical structure on Salmonella prevalence.
Feed as a source of Salmonella
It is well recognized that animal feeds and feedstuffs may be contaminated with salmonellae (Table 1).19,41,68-70 It has been demonstrated in experimental settings that animals may become infected as a result of consuming contaminated feed.70 There is no doubt that appropriate process control and decontamination steps are needed during feed processing to reduce contamination of feedstuffs and thus avoid dissemination of contaminated feed to herds.
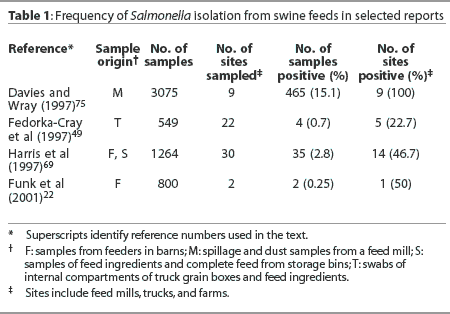
There is justification for questioning the relative importance of the role of contaminated feed in the epidemiology of Salmonella infection on swine farms. Most notably, Salmonella Typhimurium, a serovar often associated with foodborne disease in humans, is infrequently isolated from animal feeds in the United States or elsewhere.21,41,69 In a multi-country survey in Europe, salmonellae were isolated from feedstuffs in 17.6% of herds and 6.9% of all samples.21 However, the Salmonella serovars isolated from the feeds were not the same as those isolated from pigs on the farms.
Feed components and physical structure: Dry, fine, and pelleted
Epidemiological investigations, predominantly in Europe, have repeatedly demonstrated that feed composition and structure are associated with Salmonella prevalence in pigs. Among factors that have been identified are feeding either wet or dry diets, acidified diets (feed, water, or both), feed particle size, feed form (pelleted or meal), and heat-treated or non-heat-treated feeds, as well as actual feed ingredients.
Many investigators have reported that swine herds fed dry diets were at increased risk of high Salmonella seroprevalence.21,23,34,71 It is important to note that wet feeding in Europe often includes a fermentation step or addition of organic acids to prevent feed spoilage. In fact, trough feeding (adding water to feed with no preservation step) was associated with an increased risk of having a Salmonella-positive culture from pooled fecal samples in swine herds in the Netherlands.71
Whey feeds and acidifiers
A liquid whey product is usually used in diets containing whey, and is often the liquid used in fermented feed. In 1987, van Schie and Overgoor72 reported that in swine herds in the Netherlands in which liquid whey was fed as part of the diet, prevalence of Salmonella was lower than in herds in which the feed was moistened with water. In another study,21 herds fed whey were at decreased risk of being seropositive. Investigators hypothesized that a component of this effect may be related to the acidic pH of whey. Interventions using organic acids to decrease the pH of pig feedstuffs or water, mimicking the effect of whey, have had varied results in field intervention trials. In one peer-reviewed study,73 Salmonella seroprevalence was significantly lower when organic acids were administered in the drinking water in one of three herds that received proper administration of the acidified water, while in the other two herds there was a trend toward lower seroprevalence that was not statistically significant. The limited clinical trials regarding the impact of organic acid administration have been predominantly published in nonrefereed scientific meeting proceedings.
It is uncertain whether feeding organic acids is an efficacious intervention for Salmonella control on swine farms. At best, current knowledge suggests that it is of variable benefit. Although organic acids may be corrosive to metal and concrete, addition of organic acids to dry feeds or water sources may be more easily accomplished within the current US feed manufacturing and delivery infrastructure than by implementing fermented liquid feeding systems. Further evaluation of the effects of acidic pH and fermented feeds on Salmonella infection prevalence is needed to evaluate their effects within commercial production settings.
Pelleting of diets and particle size
Pelleting of feed has long been recommended as a means of decontaminating pig feeds.74 However, pelleting must be appropriately conducted, especially during the cooling phase of production, for all organisms to be destroyed.75,76 Contrary to these recommendations, epidemiological studies have found that pigs fed pelleted rations were at increased risk of high Salmonella seroprevalence compared to those fed diets in meal form.21
Investigators have hypothesized that the biological mechanism responsible for higher Salmonella infection prevalence in pigs fed pelleted feeds (compared to those fed meal feeds) may be a result of smaller particle size, heat treatment, or the pelleted form. Few peer-reviewed clinical trials investigating the effect of feed form have been published. Further work is necessary to evaluate the mechanism behind the higher Salmonella prevalence associated with pelleted feeds.
Environmental temperature and season
Groups of North Carolina finisher pigs with high Salmonella prevalence were at greater odds of having been sampled in winter and spring (approximately late November through late June).22 In the same study, pigs reared during periods of wide variation in daily high temperatures were at greater risk of high Salmonella prevalence.22 These results are similar to others in which increased seroprevalence was reported during the fall and winter in Danish swine.77
Cool weather ventilation of swine barns is a compromise between maintaining adequate air exchange while conserving heat, which may result in periods when ventilation is not optimal. Unpredictability of weather conditions makes proper setting of ventilation systems difficult. Improper ventilation or temperature stress might be a biological explanation for the association of weather with Salmonella prevalence. What makes further evaluation of this risk factor promising is that there are advantages to production performance and pig health when proper ventilation is maintained in swine buildings, which could help offset extra costs associated with improved ventilation engineering and management.
Stocking density and marketing group effects
In a study of US swine, groups of finisher pigs categorized as having high Salmonella prevalence were more likely to be stocked at higher pig densities (ie, less space allowance per pig) at the time of sampling, compared to low prevalence groups.22 In this study, initial stocking density was standardized by farm standard operating procedures, so the variation at the time of finisher sampling was accounted for by the number of pigs that had been marketed prior to sampling. One possible explanation for this finding is that transmission or shedding of salmonellae is reduced among pigs housed at lower densities, due either to decreased pig-to-pig contact or decreased stress. Alternatively, if the initial infection occurs at approximately the same time for all pigs in a barn, pigs that remain on the farm longer (because they were initially a lighter weight or grew more slowly and therefore were marketed later) had more time to recover from the infection prior to slaughter. Stocking density has known impacts on growth performance in swine,78-81 but data regarding animal density and marketing group as risk factors for shedding of salmonellae are sparse. Linton et al27 identified higher prevalence of infection in pens with higher pig density, but this result was not confirmed on subsequent sampling in the same herd. Morrow et al82 also reported a lower isolation prevalence of salmonellae in cecal contents at slaughter in pigs in older marketing groups. Further investigation is needed to evaluate the effect of stocking density and marketing group on Salmonella prevalence in swine. Potential interventions might include altering stocking density in finisher units or segregation of different marketing groups at slaughter, according to the risk of Salmonella infection. It is also critical to evaluate the effect of timing of sampling in order to standardize the measurement of Salmonella prevalence for future research investigations, since the determination of a herd's Salmonella status may vary with the timing of sampling.
Herd health status
Several authors have described lower risk of Salmonella infection in herds considered to be of high health status, usually defined by membership in specific pathogen free programs or membership in quality assurance programs that verify that certain management practices are conducted.51,71 There have also been reports that herds experiencing diarrhea outbreaks during the growing phase were at increased risk for Salmonella infection.83 In French swine herds, the odds for finisher swine to shed salmonella in the feces were greater if the pigs were seropositive for Lawsonia intracellularis or porcine reproductive and respiratory syndrome virus.34 In a study of US swine, groups of finisher pigs with high Salmonella prevalence were more likely than low prevalence groups to have above-median feed conversion rates.22
These associations of Salmonella prevalence with health status may reflect the overall expertise and management skills of the pork producer, and it is difficult to hypothesize an exact mechanism, since so many management factors may be different in high health herds compared to conventional herds. The promising aspect of these associations is that if management practices that allow for high health status designation on swine farms are also associated with decreased risk of Salmonella infection, there will be economic rewards for these producers due to improved production performance even if market benefits are not available for Salmonella control.
Implications
- The epidemiology of Salmonella infection on swine farms is complex, and further research regarding control measures is needed.
- Cost effectiveness of any intervention on the farm, or at any level of the "farm-to-fork" continuum, must be considered in order to optimally utilize resources for reduction of Salmonella contamination.
- Presently, the most practical recommendation for Salmonella control on swine farms is to implement "good management practices" for disease control.
Acknowledgement
This work was supported by the National Pork Board.
References
1. Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Inf Dis [serial online]. 1999;5:607-625. Available at: http://www.cdc.gov/ncidod/EID/eid.htm. Accessed June 10, 2004.
2. National Center for Health Statistics. Healthy People 2010. United States Departments of Health and Human Services. Available at: http://www.healthypeople.gov/document/HTML/Volume1/10Food.htm . Accessed June 22, 2004.
3. McDonagh VP, Smith HG. The significance of the abattoir in Salmonella infection in Bradford. J Hyg. 1958;56:271-279.
4. Newell KW, McClarin R, Murdock CR, MacDonald WN, Hutchinson HL. Salmonellosis in Northern Ireland, with special reference to pigs and Salmonella contaminated pig meal. J Hyg Camb. 1959;57:92-105.
5. Kampelmacher EH, Guinee PAM, Hofstra K, van Keulen A. Studies on Salmonella in slaughter-houses. Zbl Vet Med B. 1961;8:1025-1041.
6. Guinee PAM, Kampelmacher EH, Hofstra K, van Keulen A. Salmonellae in young piglets in the Netherlands. Zbl Vet Med B. 1964;12:250-256.
7. Williams LP, Newell KW. Sources of Salmonellas in market swine. J Hyg Camb. 1968;66:281-293.
8. Lee JA, Ghosh AC, Mann PG, Tee GH. Salmonellas on pig farms and in abattoirs. J Hyg Camb. 1972;70:141-150.
9. Ghosh AC. An epidemiological study of the incidence of Salmonellas in pigs. J Hyg Camb. 1972;70:151-160.
10. Harvey RWS, Price TH, Morgan J. Salmonella surveillance with reference to pigs - Cardiff abattoir, 1968-1975. J Hyg Camb. 1977;78:439-448.
11. Williams DR, Hunter D, Binder J, Hough E. Observations on the occurrence of Salmonella Choleraesuis and other Salmonellas in two herds of feeder pigs. J Hyg Camb. 1981;86:369-377.
12. Oosterom J, Notermans S. Further research into the possibility of Salmonella-free fattening and slaughter pigs. J Hyg Camb. 1983;91:59-69.
13. Davies PR, Morrow WEM, Jones FT, Deen J, Fedorka-Cray PJ, Harris IT. Prevalence of Salmonella in finishing swine raised in different production systems in North Carolina, USA. Epidemiol Infect. 1997;119:237-244.
14. United States Department of Agriculture, Animal and Plant Inspection Service. Shedding of Salmonella by finisher hogs in the U. S. Veterinary Services, National Animal Health Monitoring System; 1997. Info Sheet N223:196.
15. US Meat Export Federation. Historical Pork Export Value. Available at: http://usmef.org/Statistics2003/Historical/03_Historical_Pork Value.pdf. Accessed June 22, 2004.
16. Davies PR. Food safety and its impact on domestic and export markets. Swine Health Prod. 1997;5:13-20.
17. Alban L, Stege H, Dahl J. The new classification system for slaughter pig herds in the Danish Salmonella surveillance program. Prev Vet Med. 2002;53:133-146.
18. Pathogen reduction; hazard analysis and critical control point (HACCP) systems; final rule. Federal Regist. 1996;61:38805-38855.
19. Food Safety and Inspection Service. US Department of Agriculture. Progress Report on Salmonella Testing of Raw Meat and Poultry Products, 1998-2001. Available at: http://www.fsis.usda.gov/OPHS/haccp/salm4year.htm. Accessed June 10, 2004.
20. Berends BR, Urlings HAP, Snijders JMA, Van Knapen F. Identification and quantification of risk factors in animal management and transport regarding Salmonella spp. in pigs. Int J Food Microbiol. 1996;30:37-53.
21. Lo Fo Wong DMA, Dahl J, Stege H, van der Wolf PJ, Leontides L, von Altrock A, Thorberg BM. Herd-level risk factors for subclinical Salmonella infection in European finishing-pig herds. Prev Vet Med. 2004;62:253-266.
22. Funk JA, Davies PR, Gebreyes WA. Risk factors associated with Salmonella enterica prevalence in three-site production systems in North Carolina, USA. Berliner und Münchener Tierärztliche Wochenschrift. 2001;114:335-338.
23. van der Wolf PJ, Wolbers WB, Elbers ARW, van der Heijden HMJF, Koppen JMCC, Hunneman WA, van Schie FW, Tielen MJM. Herd level husbandry factors associated with the serological Salmonella prevalence in finishing pig herds in The Netherlands. Vet Microbiol. 2001;78:205-219.
24. Amass SF, Stevenson GW, Anderson C, Groye LA, Dowell C, Vyverberg BD, Kanitz C, Ragland D. Investigation of people as mechanical vectors for porcine reproductive and respiratory syndrome virus. Swine Health Prod. 2000;8:161-166.
25. Otake S, Dee SA, Rossow KD, Deen J, Joo HJ, Molitor TW, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by fomites (boots and coveralls). Swine Health Prod. 2002;10:59-65.
26. Davies PR, Morrow WEM, Jones FT, Deen J, Fedorka-Cray PJ, Gray JT. Risk of shedding Salmonella organisms by market-age hogs in a barn with open-flush gutters. JAVMA. 1997;210:386-389.
27. Linton AH, Heard TW, Grimshaw JJ, Pollard P. Computer-based analysis of epidemiological data arising from salmonellosis in pigs. Res Vet Sci. 1970;2:523-532.
28. Zecha BC, McCapes RH, Dungan WM, Holte RJ, Worcester WW, Williams JE. The Dillon beach project - a five-year epidemiological study of naturally occurring Salmonella infection in turkeys and their environment. Avian Dis. 1977;21:141-159.
29. Lahellec C, Colin P, Bennejean G, Paquin J, Guillerm A, Debois JC. Influence of resident Salmonella on contamination of broiler flocks. Poultry Sci. 1986;65:2034-2039.
30. Bailey JS. Control of Salmonella and Campylobacter in poultry production. A summary of work at Russell Research Center. Poultry Sci. 1993;72:1169-1173.
31. Caldwell DJ, Hargis BM, Corrier DE, Vidal L, DeLoach JR. Evaluation of persistence and distribution of Salmonella serotype isolation from poultry farms using drag-swab sampling. Avian Dis. 1995;39:617-621.
32. Baggesen DL, Wegener HC, Bager F, Stege H, Christensen J. Herd prevalence of Salmonella enterica infections in Danish slaughter pigs determined by microbiological testing. Prev Vet Med. 1996;26:201-213.
33. Hoover NJ, Kenney PB, Amick JD, Hypes WA. Preharvest sources of Salmonella colonization in turkey production. Poultry Sci. 1997;76:1232-1238.
34. Bell PA, Fravalo P, Fablet C, Jolly JP, Eveno E, Hascoet Y, Chauvin C, Salvat G, Madec F. Risk factors for Salmonella enterica subsp. enterica shedding by market-age pigs in French farrow-to-finish herds. Prev Vet Med. 2004:63:103-120.
35. McLaren IM, Wray C. Epidemiology of Salmonella Typhimurium infection in calves: persistence of Salmonellae on calf units. Vet Rec. 1991;129:461-462.
36. Plym-Forshell L, Eskebo I. Survival of Salmonellas in urine and dry faeces from cattle - an experimental study. Acta Vet Scand. 1996;37:127-131.
37. Davies RH, Wray C. Observations on disinfection regimens used on Salmonella enteritidis infected poultry units. Poultry Sci. 1995;74:638-647.
38. Davies RH, Wray C. Studies of contamination of three broiler breeder houses with Salmonella enteritidis before and after cleansing and disinfection. Avian Dis. 1996;40:626-633.
39. Madec F, Humbert F, Salvat G, Maris P. Measurement of the residual contamination of post-weaning facilities for pigs and related risk factors. J Vet Med B. 1999;46:37-45.
40. Rose N, Beaudeau F, Drouin P, Toux JY, Rose V, Colin P. Risk factors for Salmonella enterica subsp. enterica contamination in French broiler-chicken flocks at the end of the rearing period. Prev Vet Med. 1999;39:265-277.
41. Funk JA, Davies PR, Nichols MA. Longitudinal study of Salmonella enterica in two, three-site production systems. Vet Microbiol. 2001;83:45-60.
42. Clark LK, Scheidt AB, Armstrong CH, Knox K, Mayrose VB. The effect of all-in/all-out management on pigs from a herd with enzootic pneumonia. Vet Med. 1991;86:946-951.
43. Clark LK, Hill MA, Kniffen TS, VanAlstine W, Stevenson G, Meyer KB, Wu CC, Scheidt AB, Knox K, Albregts S. An evaluation of the components of medicated early weaning. Swine Health Prod. 1994;2(3):5-11.
44. Harris DL. Alternative approaches to eliminating endemic diseases and improving performance of pigs. Vet Rec. 1988;123:422-423.
45. Stege H, Christensen J, Nielsen JP, Willeberg P. Data-quality issues and alternative variable-screening methods in a questionnaire-based study on subclinical Salmonella enterica infection in Danish pig herds. Prev Vet Med. 2001;48:35-54.
46. Keteran K, Brown J, Shotts EB. Salmonella in the mesenteric lymph nodes of healthy sows and hogs. Am J Vet Res. 1982;43:706-707.
47. Davies PR, Funk JA, Morrow M. Fecal shedding of Salmonella by gilts before and after introduction to a swine breeding farm. Swine Health Prod. 2000;8:25-29.
48. Dahl J, Wingstrand A, Nielsen B, Baggesen DL. Elimination of Salmonella typhimurium infection by the strategic movement of pigs. Vet Rec. 1997;140:679-681.
49. Fedorka-Cray PJ, Harris DL, Whipp SC. Using isolated weaning to raise Salmonella-free swine. Vet Med. 1997;92:375-382.
50. Nietfeld JC, Feder I, Kramer TT, Schoneweis D, Chengappa MM. Preventing Salmonella infection in pigs with offsite weaning. Swine Health Prod. 1998;6:27-32.
51. Kranker S, Dahl J, Wingstrand A. Bacteriological and serological examination and risk factor analysis of Salmonella occurrence in sow herds, including risk factors for high Salmonella seroprevalence in receiver finishing herds. Berliner und Münchener Tierärztliche Wochenschrift. 2001;114:350-352.
52. Renwick SA, Irwin RJ, Clarke RC, McNab WB, Poppe C, McEwen SA. Epidemiological associations between characteristics of registered broiler chicken flocks in Canada and the Salmonella culture status of floor litter and drinking water. Can Vet J. 1992;33:449-458.
53. Henken AM, Frankena K, Goelema JO, Graat EAM, Noordhuizen JPTM. Multivariate epidemiological approach to salmonellosis in broiler breeder flocks. Poultry Sci. 1992;71:838-843.
54. Kabagambe EK, Wells SJ, Garber LP, Salman MD, Wagner B, Fedorka-Cray PJ. Risk factors for fecal shedding of Salmonella in 91 US dairy herds in 1996. Prev Vet Med. 2000;43:177-194.
55. Barber DA, Weigel RM, Isaacson RE, Bahnson PB, Jones CJ. Distribution of Salmonella in swine production ecosystems. J Food Protect. 2002;65:1861-1868.
56. Henzler DJ, Opitz HM. The role of mice in the epizootiology of Salmonella enteritidis infection on chicken layer farms. Avian Dis. 1992;36:625-631.
57. Guard-Petter J, Henzler DJ, Mahbubur Rahman M, Carlson RW. On-farm monitoring of a mouse-invasive Salmonella enterica serovar enteritidis and a model for its association with the production of contaminated eggs. Appl Environ Microbiol. 1997;63:1588-1593.
58. Davies RH, Wray C. Mice as carriers of Salmonella enteriditis on persistently infected poultry units. Vet Rec. 1995;137:337-341.
59. Davies R, Breslin M. Environmental contamination and detection of Salmonella enterica serovar enteriditis in laying flocks. Vet Rec. 2001;149:699-704.
60. Liebana E, Garcia-Migura L, Clouting C, Clifton-Hadley FA, Breslin M, Davies RH. Molecular fingerprinting evidence of the contribution of wildlife vectors in the maintenance of Salmonella Enteriditis in layer farms. J Appl Microbiol. 2003;94:1024-1029.
61. Johnston WS, Maclachlan GK, Hopkins GF. The possible involvement of seagulls (Larus larus) in the transmission of salmonella in dairy cattle. Vet Rec. 1979;105:526-527.
62. Butterfield J, Coulson JC, Kearsey SV, Monaghan P, McCoy JH, Spain GE. The herring gull Larus argentatus as a carrier of salmonella. J Hyg. 1983;91:429-436.
63. Coulson JC, Butterfield J, Thomas C. The herring gull Larus argentatus as a likely transmitting agent of Salmonella Montevideo to sheep and cattle. J Hyg. 1983;91:437-443.
64. Fenlon DR. A comparison of salmonella serotypes found in the feces of gulls feeding at a sewage works with serotypes present in the sewage. J Hyg. 1983;91:47-52.
65. Craven SE, Stern NJ, Line E, Bailey JS, Cox NA, Fedorka-Cray P. Determination of the incidence of Salmonella spp., Campylobacter jejuni, and Clostridium perfringens in wild birds near broiler chicken houses by sampling intestinal droppings. Avian Dis. 2000;44:715-720.
66. Goodwin MA, Waltman WD. Transmission of Eimeria, viruses, and bacteria to chicks: darkling beetles (Alphitobius diapernus) as vectors of pathogens. J Appl Poultry Sci. 1996;5:51-55.
67. Aballay A, Yorgey P, Ausubel M. Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol. 2000;10:1539-1542.
68. Fedorka-Cray PJ, Hog A, Gray JT, Lorenzen K, Velasquez J, Von Behren P. Feed and feed trucks as sources of Salmonella contamination in swine. Swine Health Prod. 1997;5:189-193.
69. Harris IT, Fedorka-Cray PJ, Gray JT, Thomas LA, Ferris K. Prevalence of Salmonella organisms in swine feed. JAVMA. 1997;210:382-385.
70. Williams Smith H. The effect of feeding food naturally contaminated with salmonellae. J Hyg Camb. 1960;58:381-389.
71. van der Wolf PJ, Bongers JH, Elbers ARW, Franssen FMMC, Hunneman WA, van Exsel ACA, Tielen MJM. Salmonella infections in finishing pigs in The Netherlands: bacteriological herd prevalence, serogroup and antibiotic resistance of isolates and risk factors for infection. Vet Microbiol. 1999;67:263-275.
72. van Schie FW, Overgoor GH. An analysis of the possible effects of different feed upon the excretion of salmonella bacteria in clinically normal groups of fattening pigs. Vet Q. 1987;9:185-188.
73. van der Wolf PJ, van Schie FW, Elbers AR, Engel B, van der Heijden HM, Hunneman WA, Tielen MJ. Administration of acidified drinking water to finishing pigs in order to prevent Salmonella infections. Vet Q. 2001;121-125.
74. Edel W, Guinee PAM, van Schothorst M, Kampelmacher EH. Salmonella infections in pigs fattened with pellets and unpelleted meal. Zbl Vet Med B. 1970;14:393-401.
75. Davies RH, Wray C. Distribution of Salmonella contamination in ten animal feed mills. Vet Microbiol. 1997;51:159-169.
76. Himathongkham S, das Gracas Pereira M, Riemann H. Heat destruction of Salmonella in poultry feed: effect of time, temperature, and moisture. Avian Dis. 1996;40:72-77.
77. Christensen J, Rudemo M. Multiple change-point analysis applied to the monitoring of Salmonella prevalence in Danish pigs and pork. Prev Vet Med. 1998;36:131-143.
78. Randolph JH, Cromwell GL, Stahly TS, Kratz DD. Effects of group size and space allowance on performance and behavior of swine. J Anim Sci. 1981;53:922-927.
79. Kornegay ET, Lindemann MD, Ravindran V. Effects of dietary lysine levels on performance and immune response of weanling pigs housed at two floor space allowances. J Anim Sci. 1993;71:552-556.
80. Kornegay ET, Meldrum JB, Chickering WR. Influence of floor space allowance and dietary selenium and zinc on growth performance, clinical pathology, and adrenal weights of weanling pigs. J Anim Sci. 1993;71:3185-3198.
81. Hyun Y, Ellis M, Riskowski G, Johnson RW. Growth performance of pigs subjected to multiple concurrent environmental stressors. J Anim Sci. 1998;76:721-727.
82. Morrow WE, See MT, Eisemann JH, Davies PR, Zering K. Effect of withdrawing feed from swine on meat quality and prevalence of Salmonella colonization at slaughter. JAVMA. 2002;220:497-502.
83. Møller K, Jensen TK, Jorsal SE, Leser TD, Carstensen B. Detection of Lawsonia intracellularis, Serpulina hyodysenteriae, weakly beta-hemolytic intestinal spirochetes, Salmonella enterica, and haemolytic Escherichia coli from swine herds with and without diarrhea among growing pigs. Vet Microbol. 1998;62:59-72
