Original research |
Peer reviewed |
Effects of in-feed antimicrobial alternatives and antimicrobials on nursery pig performance and weight variation
Efectos de varias alternativas antimicrobianas y antimicrobianos para el alimento en el desempeño de los cerdos en el destete y la variación de peso
Les effets de diverses alternatives antimicrobiennes, et antimicrobiennes en la moulée dans la performance de porcelets dans la pouponnière et variation du poids
Timothy P. Keegan, MS; Steve S. Dritz, DVM, PhD; Jim L. Nelssen, PhD; Joel M. DeRouchey, PhD; Mike D. Tokach, PhD; Robert D. Goodband, PhD
TPK, JLN, JMDR, MDT, RDG: Department of Animal Sciences and Industry, Kansas State University, Manhattan, Kansas; SSD: Food Animal Health and Management Center, College of Veterinary Medicine, Kansas State University, Manhattan, Kansa; Corresponding author: Dr Steve S. Dritz, Food Animal Health and Management Center, College of Veterinary Medicine, Kansas State University, Manhattan, KS 66506; Tel: 785-532-4202; Fax: 603-676-5543; E-mail: dritz@vet.ksu.edu
Cite as: Keegan TP, Dritz SS, Nelssen JL, et al. Effects of in-feed antimicrobial alternatives and antimicrobials on nursery pig performance and weight variation. J Swine Health Prod. 2005;13(1):12-18.
Also available as a PDF.
SummaryObjectives: To compare the effects of several antimicrobial alternatives and in-feed antimicrobials on nursery pig performance. Methods: In Experiment One, 720 nursery pigs were used to evaluate feeding three yeast products (LactoSacc, Alltech, Nicholasville, Kentucky; Biomate Yeast Plus, Chr Hansen BioSystems, Milwaukee, Wisconsin; BioSaf, Saf Agri, Minneapolis, Minnesota); three antimicrobial products containing bacteria (Probios and Bio-Plus 2B, Chr Hansen BioSystems) or oligosaccharides (Bio-Mos, Alltech); and the antimicrobial carbadox (Phibro Animal Health, Fairfield, New Jersey). In Experiment Two, 320 nursery pigs were used to evaluate feeding LactoSacc, Bio-Plus 2B, Bio-Mos, and carbadox in an on-farm trial. In Experiment Three, 320 nursery pigs (same commercial herd as in Experiment Two) were used to evaluate feeding carbadox, a combination of tiamulin (Boehringer Ingelheim Vetmedica, St Joseph, Missouri) and chlortetracycline (Alpharma, Fort Lee, New Jersey), a combination of neomycin and oxytetracycline (Phibro Animal Health), and Bio-Mos. Results: In Experiment One, in pigs fed the carbadox diet, ADG was greater (P < .05) than in pigs fed all other diets, and feed:gain was better (P < .05) than in pigs fed the control diet or the Probios diet. In Experiment Two, performance did not differ among treatment groups and controls. In Experiment Three, pigs fed the tiamulin-chlortetracycline and neomycin-oxytetracycline diets had greater ADG and average daily feed intake compared with control pigs (P < .05). There were no differences in growth performance among control pigs and those fed the carbadox or Bio-Mos diets. Implication: The antimicrobial alternatives evaluated did not enhance growth performance. | ResumenObjetivos: Comparar los efectos de varias alternativas antimicrobianas y antimicrobianos para alimento en el desempeño de los cerdos en el destete. Métodos: En el Experimento Uno, 720 cerdos de destete fueron utilizados para evaluar la alimentación de tres productos de levaduras (LactoSacc, Alltech, Nicholasville, Kentucky; Biomate Yeast Plus, Chr Hansen BioSystems, Milwaukee, Wisconsin; BioSaf, Saf Agri, Minneapolis, Minnesota); tres productos antimicrobianos que contienen bacterias (Probios and Bio-Plus 2B, Chr Hansen BioSystems) u oligosacaridos (Bio-Mos, Alltech); y el antimicrobiano carbadox (Phibro Animal Health, Fairfield, New Jersey). En el Experimento Dos, 320 cerdos de destete fueron utilizados para evaluar la alimentación con LactoSaac, Bio-Plus 2B, Bio-Mos y carbadox en una prueba de granja. En el Experimento Tres, 320 cerdos de destete (de la misma granja comercial que en el Experimento Dos) fueron utilizados para evaluar la alimentación con carbadox, una combinación de tiamulina (Boehringer Ingelheim Vetmedica, St Joseph, Missouri) y clorotetraciclina (Alpharma, Fort Lee, New Jersey), una combinación de neomicina y oxitetracilina (Phibro Animal Health) y Bio Mos. Resultados: En el Experimento Uno, en cerdos alimentados con la dieta de carbadox, la GDP fue mayor (P < .05) que en los cerdos alimentados con las otras dietas; y la relación alimento:ganancia fue mejor (P < .05) que en los cerdos alimentados con la dieta control o la dieta de Probios. En el Experimento Dos, el desempeño no varió entre los grupos tratados y los controles. En el Experimento Tres, los cerdos que recibieron los alimentos que contenían tiamulina-clorotetracilina y neomicina-oxitetracilina tuvieron una mayor GDP y un consumo de alimento diario promedio mayor comparado con los cerdos control (P < .05). No hubo diferencias en el desempeñó de crecimiento entre los cerdos control y aquellos alimentados con las dietas de carbadox o de Bio Mos. Implicación: Las alternativas antimicrobianas evaluadas no mejoraron el desempeño del crecimiento. | ResuméObjectifs: Comparer les effets de diverses alternatives antimicrobiennes, et antimicrobiennes en la moulée dans la performance de porcelets dans la pouponnière. Méthodes: Dans le Expérience Un, 720 porcelets dans la pouponnière ont été utilisées pour évaluer la alimentation de trois produits de levures (LactoSaac, Alltech, Nicholasville, Kentucky; Biomate Yeast Plus, Chr Hansen BioSystems, Milwaukee, Wisconsin; BioSaf, Saf Agri, Minneapolis, Minnesota); trois produits antimicrobiens qui contiennent bactéries (Probios et Bio-Plus 2B, Chr Hansen BioSystems) ou oligosaccharides (Bio-Mos, Alltech); et le antimicrobien carbadox (Phibro Animal Health, Fairfield, New Jersey). Dans le Expérience Deux, 320 porcelets dans la pouponnière ont été utilisés pour évaluer la alimentation avec LactoSaac, Bio-Plus 2B, Bio-Mos y carbadox en une essai dans la ferme. Dans le Expérience Trois, 320 porcelets dans la pouponnière (le même troupeau que dans le Expérience Deux)ont été utilisés pour évaluer la alimentation avec carbadox, une combinaison de tiamulin (Boehringer Ingelheim Vetmedica, St Joseph, Missouri) et chlorotétracycline (Alpharma, Fort Lee, New Jersey), une combinaison de néomycine et oxitétracycline (Phibro Animal Health), et Bio Mos. Résultats: Dans le Expérience Un, les porcelets nourris avec le régime de carbadox, le GMQ a été plus grand (P < .05) que les porcelets nourris avec des autres régimes; et la relation moulée:croissance a été meilleur (P < .05) que les porcelets nourris avec le régime control ou le régime de Pro bios. Dans le Expérience Deux, la performance n'a pas différé parmi les groups de traitement et de contrôle. Dans le Expérience Trois, les porcelets nourris les régimes de tiamulin-chlorotétracycline et néomycine-oxitétraycline ont eu un GMQ et une consumation moyenne par jour plus grands comparé á les porcelets contrôle (P < .05). Il n'y a pas eu des différences de croissance dans les porcelets de contrôle et celles nourris les régimes de carbadox ou de Bio Mos. Implication: Les alternatives antimicrobiennes évaluées n'ont pas amélioré la performance de la croissance. |
Keywords: swine, nursery,
antibiotic,
antimicrobial, growth
Search the AASV web site
for pages with similar keywords.
Received: July
3, 2004
Accepted: September
10, 2004
In-feed antimicrobials have been widely used within the swine industry to prevent disease and promote growth rate and feed efficiency.1,2 The use of in-feed antimicrobials has long been recognized as an effective management practice to improve pig performance.1,2 Summaries conducted in 1978 by Hays1 and from 1979 to 1985 by Zimmerman2 found an improvement in nursery pigs of 16.4% in daily gain and 6.9% improvement in feed efficiency. It has been reported, in the United States,3 that antimicrobials are included in 90% of starter diets, 75% of grower diets, and more than 50% of finishing diets. However, because of changes in modern swine production, there is a need to re-analyze the use of in-feed antimicrobials throughout all stages of production.4 There also have been concerns that feeding antibiotics to food animals may affect human health.3 The potential for agricultural antibiotics to contribute to development of antibiotic-resistant bacteria in humans is the subject of intense debate and research.5 Several countries have implemented strict guidelines and regulations on the use of in-feed antimicrobials for production purposes. Even though the National Research Council3 conducted a thorough review of the literature and concluded that feeding antibiotics to animals does not constitute an immediate concern in public health, there has been interest in researching antibiotic alternatives.
Overall, antimicrobials are used in swine production for their ability to suppress or inhibit growth of certain microorganisms,6 while antibiotic alternatives are intended to modify the gastrointestinal microflora in such a way that activities beneficial to the host are stimulated and those adverse to host health are suppressed.7 Possible modes of action include alteration in the composition of intestinal microflora to suppress specific groups of organisms, alteration of microbial metabolism, and stimulation of immunity.8 But results of studies with pigs fed diets containing supplemental yeast, direct-fed microbials, and mannan oligosaccharides have been conflicting, with some trials showing no improvement,5 whereas others show benefits in growth performance.9,10 Many of these studies are limited in scope or do not contain direct comparisons to in-feed antimicrobials.
Our original objective was to evaluate the use of several feed additives in research and commercial settings. Because of a lack of production response to the in-feed antimicrobial carbadox in the commercial environment, a secondary objective was to compare growth performance in pigs fed carbadox and other in-feed antimicrobials in this facility.
Materials and Methods
Pigs, housing, measures of growth performance, and diets
The Kansas State University Institutional Animal Care and Use Committee approved all experimental protocols used in this study.
Pigs (PIC, Franklin, Kentucky) were housed in environmentally controlled nursery facilities with slatted metal flooring and mechanical ventilation. In Experiment One, pigs were housed five per pen, with one self-feeder and one nipple waterer in each 1.44-m2 pen. In Experiments Two and Three, pigs were housed eight per 2.16-m2 pen, with one self-feeder and two nipple waterers in each pen providing ad libitum access to feed and water.
All pigs were randomly assigned to treatments at weaning. Average daily gain (ADG), average daily feed intake (ADFI), feed efficiency (F:G), and within-pen coefficient of variation of body weight (CV) were determined by weighing individual pigs and measuring pen feed disappearance on days 7, 14, 21, and 27 postweaning in Experiment One; on days 10, 16, 23, and 31 postweaning in Experiment Two; and on days 9, 22, and 31 postweaning in Experiment Three.
All experimental diets were based on corn and soybean meal, with added specialty ingredients as indicated, and were formulated to meet or exceed nutrient requirements suggested by the NRC11 and according to principles outlined by Nelssen et al.12 Ingredient nutrient compositions used in diet formulation were those made available by the NRC, 1998.11 Diets were manufactured at the Kansas State University feed mill under supervision of the lead author and were fed as meal. All diets were analyzed for crude protein, calcium, and phosphorus. Chemical composition was within expected analytic variance in each case. A single lot of each antimicrobial alternative was used in these experiments. Each feed additive was added to the diet at the manufacturer's recommended inclusion rates, replacing an equivalent amount of corn starch in the control diet. Feed additives were stored under conditions recommended by the manufacturer in the usage instructions.
Experiment One
A total of 720 weanling barrows (weaning weight 5.8 +/- 0.37 kg, 18 +/- 2 days of age) were blocked by weight and allotted to eight dietary treatments. The experiment was divided into two trials, the first beginning in September 2002, and the second beginning in January 2003, which were conducted at the Kansas State Segregated Early Wean Facility (Manhattan, Kansas). In each trial, there were nine replications (pens) per treatment. The results were combined for statistical analysis, with a total of 18 replications.
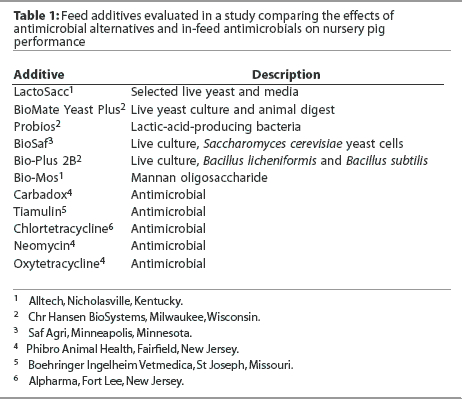
All pigs were fed experimental diets from weaning (Day 0) to Day 27. The eight experimental diets included a control diet without any additive or antimicrobial. Each of the seven experimental diets was formulated by adding to the control diet one of the antimicrobial alternatives described in Table 1. Additives and their concentrations in the final diets were as follows: carbadox, 55 mg per kg; Probios, 1.6% Days 0 to 13 and 0.8% Days 14 to 20; BioSaf, 0.3%; Biomate Yeast Plus, 0.1%; Bio-Mos, 0.3%; Bio-Plus 2B, 0.05%; and LactoSacc, 0.2%.
Phase one diets (Days 0 through 13) were formulated to contain 1.50% lysine, 0.90% calcium, and 0.54% available phosphorus, and contained 15% spray-dried whey and 5% spray-dried animal plasma. Phase two diets (Days 14 through 27) were formulated to contain 1.45% lysine, 0.85% calcium, and 0.44% available phosphorus, and contained 7.5% spray-dried whey and 2.5% select menhaden fishmeal. Phase one and two diets did not contain growth-promoting concentrations of copper or zinc.
Experiment Two
A total of 320 weanling pigs (weaning weight 5.3 +/- 0.27 kg, 14 +/- 2 days of age) were blocked by weight and allotted to five dietary treatments, with eight replicates (pens) per treatment, and with barrows and gilts equally distributed among pens. The trial was conducted at a commercial farm in Northeast Kansas.
All pigs were fed experimental diets from weaning (Day 0) to Day 31. The five experimental diets included a control diet without antimicrobials or additives. Each of the four experimental diets was formulated by adding to the control diet one of the antimicrobial alternatives described in Table 1. Additives and their concentrations in the final diets were as follows: carbadox, 55 mg per kg; LactoSacc, 0.2%; Bio-Plus 2B, 0.05%; and Bio-Mos, 0.3%. Segregated early wean (SEW) diets were fed according to a feed budget of 0.45 kg per pig and were formulated to contain 1.70% lysine, 0.81% calcium, 0.60% available phosphorus, and 3000 ppm zinc from zinc oxide. The SEW diet contained 25% spray-dried whey, 6.7% spray-dried animal plasma, 6% select menhaden fishmeal, 5% lactose, and 1.65% spray-dried blood meal. After consumption of SEW diet budget, transition diets were fed to Day 10. Transition diets were formulated to contain 1.60% lysine, 0.92% calcium, 0.59% available phosphorus, and 3000 ppm zinc from zinc oxide, and contained 25% spray-dried whey, 2.5% spray-dried animal plasma, and 6% fishmeal. Phase two diets (Days 11 through 31) were formulated to contain 1.51% lysine, 0.81% calcium, 0.47% available phosphorus, and 3000 ppm zinc from zinc oxide, and contained 10% whey and 4.5% fishmeal.
Experiment Three
A total of 320 weanling pigs (weaning weight 4.9 +/- 0.21 kg, 14 +/- 2 days of age) were blocked by weight and allotted to five dietary treatments with eight replicates (pens) per treatment, and with barrows and gilts equally distributed among pens. The trial was conducted at the same commercial facility used in Experiment Two.
All pigs were fed experimental diets from weaning (Day 0) to Day 31. The five experimental diets included a control diet without antimicrobials or additives. Each of the four experimental diets was formulated by adding to the control diet one or more of the additives described in Table 1. Additives and their concentrations in the final diets were as follows: carbadox, 55 mg per kg; tiamulin, 38 mg per kg, plus chlor-tetracycline, 441 mg per kg (Tiamulin-CTC); neomycin sulfate, 54 mg per kg, plus oxytetracycline, 154 mg per kg (Neo-Oxy); and Bio-Mos, 0.3%. Diets and feeding program were the same as in Experiment Two.
Statistical analysis
Each experiment was analyzed as a randomized complete block design with pen as the experimental unit. Pigs were blocked on the basis of weaning weight, and analysis of variance was performed using the Mixed Procedure of SAS (SAS Institute, Inc, Cary, North Carolina), with block as a random effect and treatment as a fixed effect.13 In addition, trial was included in the model as a random effect in Experiment One. Coefficient of variation (CV) was calculated as the standard deviation of within-pen pig weight divided by mean pig weight for that pen. Change in CV was calculated as the within-pen CV at the end of the experiment minus the within-pen CV at the beginning of the experiment. All means presented are least squares means, protected by significant F-tests (ie, treatment differences are reported only if the overall model P-value is < .05).
Results
Experiment One
Overall (Days 0 to 27), mean ADG was greater (P < .05) in pigs fed the diet containing carbadox than in pigs fed all other diets (Table 2), with 21.4 g the least significant detectable difference in ADG. In contrast, the largest difference in ADG among groups fed the control and antimicrobial alternative diets was 6 g. Mean ADFI was greater in pigs fed the diet containing carbadox than in pigs fed diets containing BioSaf, Yeast Plus, Bio-Mos, Bio-Plus 2B, or LactoSacc (P < .05). Mean ADFI was greater in pigs fed the diet containing Probios than in pigs fed diets containing Bio-Mos or Bio-Plus 2B (P < .05). Mean F:G was better in pigs fed the diet containing carbadox than in pigs fed the control diet or the diet containing Probios (P < .05). Mean F:G was better in pigs fed diets containing BioSaf, Yeast Plus, Bio-Mos, Bio-Plus 2B, or LactoSacc than in pigs fed the diet containing Probios (P < .05). The overall statistical model indicated differences among treatments (P = .02) for initial within-pen weight variation (Day 0 CV) as a result of greater weight variation (P < .05) in pigs fed the control diet than in pigs on all other treatments except Bio-Mos. However, no differences were observed among treatments for Day 27 CV or for the change in CV between Days 0 and 27.
Experiment Two
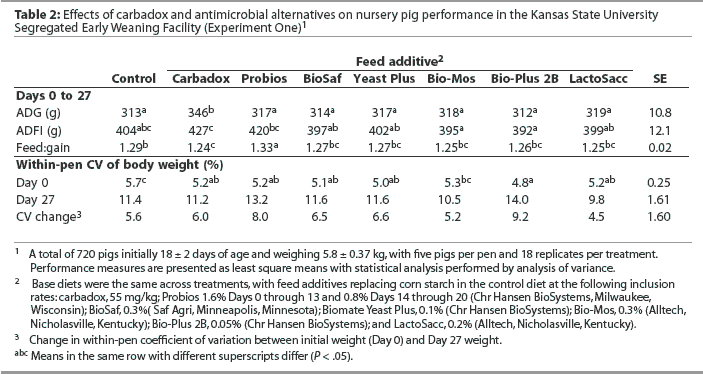
Overall (Days 0 to 31), mean ADG was greater (P < .05) in pigs fed the control diet or the diet containing Bio-Mos than in pigs fed the diet containing Bio-Plus 2B (Table 3), with 24.4 g the least significant detectable difference in ADG. Mean ADFI was lower (P < .05) in pigs fed the diet containing Bio-Plus 2B than in pigs fed all other diets except the diet containing LactoSacc. There were no differences in feed efficiency or weight variation among treatments.
Experiment Three
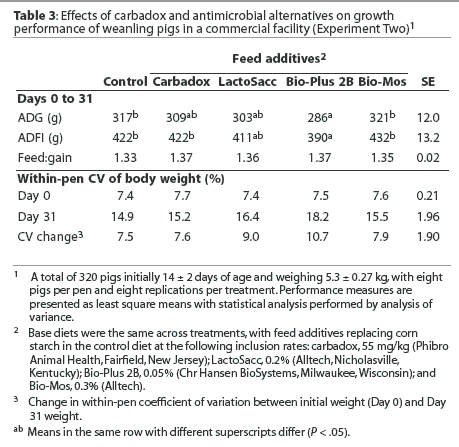
Mean ADG and ADFI were greater (P < .05) in pigs fed the diet containing Tiamulin-CTC than in pigs fed all other treatment diets (Table 4). Mean ADG was greater (P < .05) in pigs fed the diet containing Neo-Oxy than in pigs fed the control diet or pigs fed diets containing carbadox or Bio-Mos, with 22.5 g the least significant detectable difference in ADG. In addition, mean ADFI was greater (P < .05) in pigs fed the diet containing Neo-Oxy than in pigs fed the control diet or the diet containing Bio-Mos.
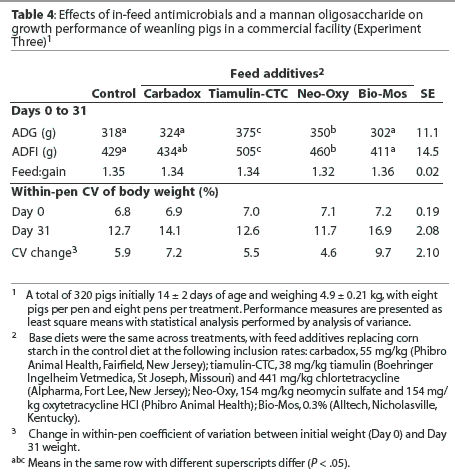
There were no differences in pig weight variation observed on Day 0, but weight variation tended to increase (P < .1) between Days 0 and 31 for pigs fed the diet containing Bio-Mos.
Discussion
Several antimicrobial alternatives have been extensively researched. Results for yeast, direct-fed microbials, and mannan oligosaccharides have been conflicting, with some studies showing improvements in growth performance10,14 and others showing no difference.5 Probiotics are defined as live microbial feed supplements, in comparison to prebiotics, which stimulate growth of only certain bacterial species already present in the host.7 All antimicrobial alternatives tested in this study are considered probiotics with the exception of the mannan oligosaccharide, which is considered a prebiotic.
A summary by Hillman15 evaluating the effectiveness of probiotics concluded that, although they may be efficient at growth promotion in pigs, the consistency of responses to individual strains and their activity is variable. The use of several yeast products (probiotics) has been shown beneficial to improve growth performance of weanling pigs.9,16 However, most of the responses observed varied with individual conditions within each experiment.17 A review of 49 comparisons of performance with use of a mannan oligosaccharide found increases of 4.18%, 2.14%, and 2.24% for ADG, ADFI, and feed efficiency, respectively,18 but few of these reports have been published in peer-reviewed publications.
It has been suggested that inclusion of growth-promoting amounts of copper and zinc also affect pig growth performance when prebiotic or probiotic antimicrobial alternatives are fed.19-21 Experiment One diets did not contain growth-promoting amounts of copper and zinc. In Experiments Two and Three, diets were similar to current formulations fed in the commercial facility and contained growth-promoting amounts of zinc but not copper. Results from our three experiments fail to indicate a difference in response to antimicrobial alternatives with the addition of growth-promoting amounts of zinc oxide.
Experiment One was designed to evaluate the effects of antimicrobial alternatives in a research setting. In past experiments at this research facility, improvements in nursery pig performance have been found with the addition of carbadox to the diet.22 Results of Experiment One agree with these previous results; ADG and ADFI in pigs fed a diet containing carbadox were higher by 10% and 6%, respectively, than in pigs fed the control diet. These results agree with those of Harper and Estienne,23 who found no effects with Bio-Mos supplementation and an actual reduction in growth rate with the use of Bio-Plus 2B. Several factors may contribute to the lack of response to antimicrobial alternatives. The first is the purity and degree of specificity of the organisms used in the antimicrobial alternative products.24 The number of strains of bacteria used in such feed additives and the condition of the cultures in which they are produced may affect consistency of pig growth performance. Because many antimicrobial alternatives contain live cell cultures, effectiveness depends on proper storage of the products and longevity of the cultures. All products used in these trials were evaluated within the recommended product-stability timelines provided by the manufacturers and were stored to meet manufacturer recommendations.
Changes in facilities and management, as well as pig health, might have a major impact on overall effectiveness of feed additives. It is hypothesized that the production response to antimicrobial alternatives would be greater under conditions of poorer sanitation and base level of growth performance, as is true for in-feed antimicrobials.25 To test this possibility, we conducted Experiments Two and Three on a commercial farm in Northeast Kansas. A yeast, a bacillus product, and a mannan oligosaccharide were included to ensure a representative sampling of different antimicrobial-alternative products. On the basis of Experiment One growth performance results, LactoSacc, Bio-Plus 2B, and Bio-Mos were selected for comparison with the control and carbadox. As in Experiment One, addition of antimicrobial alternatives to nursery pig diets did not result in enhancement of growth performance over that in the control group. Overall, there was no difference in feed efficiency, but ADG and feed intake were poorer in the group on the feed additive Bio-Plus 2B. Furthermore, there was no impact of any of the antimicrobial alternatives on within-pen weight variation.
We were surprised to find that addition of carbadox to the diet did not enhance performance, compared with the control diet, in Experiment Two. This commercial farm had been using carbadox as an antibacterial agent for approximately 10 years prior to this experiment. Response to feeding antimicrobial agents under field conditions has been observed to be nearly double that found under university or research conditions.1 In addition, improvement in growth performance with use of dietary antimicrobials differs at different nursery facilities.26 To accurately assess the influence of in-feed carbadox on performance, a control diet with no antimicrobials or antimicrobial alternatives was included in Experiments Two and Three.
Production methods used to raise pigs in the United States have improved during the past 15 years, with emphasis on improved hygiene. The adoption and use of multi-site pig production systems decreases vertical pathogen spread from adult to growing pigs and lateral pathogen spread among groups of pigs.4 In addition, rapid growth rate is achieved in high-lean pigs subjected to minimal disease challenge and provided with adequate housing. Because these conditions are negatively correlated with magnitude of response to growth promotants, less response would be expected in pigs that already have rapid growth rates.27 A summary of 10 trials conducted by Dritz et al4 with the use of antimicrobials in nursery and finisher pigs reported a smaller increase in pig performance with the use of in-feed antimicrobials than had been reported for earlier trials. It is suggested that use of in-feed, growth-promoting antimicrobials in multi-site pig production should be limited to therapeutic applications in finishing pigs.4 Results from Experiment Two, showing no influence of carbadox on growth performance, might also be applicable on some farms in the nursery stages of growth. Therefore, Experiment Three was designed to evaluate use of several different in-feed antimicrobials. Two antimicrobial combinations, tiamulin with chlortetracycline and neomycin with oxytetracycline, were tested, as well as carbadox, to determine if these antimicrobials would enhance growth performance. Addition of carbadox to the diet in this commercial operation did not enhance growth performance, as in Experiment Two, but growth performance was enhanced with addition of either tiamulin with chlortetracycline or neomycin with oxytetracycline, primarily from Day 10 to Day 31, when pigs were fed phase two diets. This agrees with prior data suggesting that enhancements in growth performance with use of antibiotics are seen 1 to 2 weeks after administration of antibiotics.20 Even though ADG and ADFI were higher overall with the addition of tiamulin plus chlortetracycline and with neomycin plus oxytetracycline, addition of either combination of antimicrobials did not affect weight variation on Day 31 or weight gain variation.
Further research to determine the lack of carbadox effectiveness is warranted. Although diets were not analyzed for carbadox content, diets were manufactured under the direct supervision of the primary author, who was responsible for addition of all additives. One plausible explanation for the lack of response to carbadox is an interaction between carbadox and pharmacologic levels of zinc from zinc oxide. This explanation is based on the positive response to carbadox in Experiment One, in which pharmacological levels of zinc were not included in the diets, and the lack of response in the subsequent experiments, when pharmacologic levels of zinc were provided. However, a large-scale study across seven research stations reported an additive effect of feeding carbadox and pharmacologic levels of zinc from zinc oxide.28
Implication
The yeast, probiotic, and prebiotic antimicrobial alternatives tested in nursery pigs were less effective in growth promotion than in-feed antimicrobials.
Contribution No. 04-440-J from the Kansas Agricultural Experiment Station, Kansas State University, Manhattan, KS 66506.
References
1. Hays VW. Effectiveness of Feed Additive Usage of Antibacterial Agents in Swine and Poultry Production. Report to the Office of Technology Assessment. Washington, DC: US Government Printing Office; 1978.
*2. Zimmerman DR. Role of subtherapeutic levels of antimicrobials in pig production. J Anim Sci. 1985;62(suppl 3):6-17.
3. National Research Council. Use of Drugs in Food Animals: Benefits and Risks. Washington, DC: National Academy Press; 1999.
4. Dritz SS, Tokach MD, Goodband RD, Nelssen JL. Effects of administration of antimicrobials in feed on growth rate and feed efficiency of pigs in multisite production systems. JAVMA. 2002;220:1690-1695.
5. Doyle E. Alternatives to antibiotic use for growth promotion in animal husbandry. Food Research Institute. April 2001.
6. Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. 2002;13(1):7-27.
7. Simmering R, Blaut M. Pro and prebiotics - the tasty guardian angels? Appl Microbiol Biotechnol. 2001;55:19-28.
8. Fuller F. Probiotics in man and animals. J Appl Bacteriol. 1989;66:365-378.
9. van Heugten E, Funderburke DW, Dorton KL. Growth performance, nutrient digestibility, and fecal microflora in weanling pigs fed live yeast. J Anim Sci. 2003;81:1004-1012.
*10. Dvorak R, Jacques KA. Mannanoligosaccharide, fructooligosaccharide and carbadox for pigs days 0 - 21 postweaning [abstract]. J Anim Sci. 1998;76(suppl 2):64. Abstract 156.
11. National Research Council. Nutrient Requirements of Swine. 10th ed. Washington, DC: National Academy Press; 1998.
12. Nelssen JL, Dritz SS, Tokach MD, Goodband RD. Diseases of Swine. 8th ed. Nutritional programs for segregated early weaning. Ames, Iowa: Iowa State University Press; 1999:1045-1055.
13. Littell RC, Stroup WW, Freund RJ. SAS for Linear Models. 4th ed. Cary, North Carolina: SAS Institute Inc; 2002:106-112.
*14. Hancock JD, Jones CL, Starkey CW. Effects of mannanoligosaccharides in diets for nursery pigs [abstract]. J Anim Sci. 2002;80(suppl 2):68. Abstract 148.
15. Hillman K. Bacteriological aspects of the use of antibiotics and their alternatives in the feed of non-ruminant animals. In: Wiseman J, Gansworthy PC, eds. Recent Development in Pig Nutrition 3. Nottingham, UK: Nottingham University Press; 2001.
*16. Maloney CA, Hancock JD, Hines RH, Cao H, Nemecek CS, Park JS. Effects of a heat-stable yeast product in pelleted diets for weanling pigs. Kansas Agr Exp Sta Progr Rep 819. 1998;819:62-66.
17. Kornegay ET, Rhein-Welker D, Lindemann MD, Wood CM. Performance and nutrient digestibility in weanling pigs as influenced by yeast culture additions to starter diets containing dried whey or one of two fiber sources. J Anim Sci. 1995;73:1381-1389.
*18. Miguel JC, Rodriguez-Zas SL, Pettigrew JE. Practical effects of Bio-Mos in nursery pig diets: a meta-analysis. In: Lyons TP, Jacques KA, eds. Proc Alltech's 18th Ann Symp; 2002.
19. Davis ME, Maxwell CV, Brown CD, de Rodas BZ, Johnson ZB, Kegley EB, Hellwig DH, Dvorak RA. Effects of dietary mannan oligosaccharides and or pharmacological additions of copper sulfate on growth performance and immunocompetence of weanling and growing/finishing pigs. J Anim Sci. 2001;80:2887-2894.
*20. Davis ME, Brown DC, Maxwell CV, Johnson ZB, Dvorak RA. Efficacy of mannan oligosaccharide (Bio-Mos) as a complete or partial replacement for zinc oxide in diets of weanling pigs [abstract]. J Anim Sci. 2001;79(suppl 2):78. Abstract 209.
*21. Davis ME, Kegley EB, de Rodas BZ, Friesen KG, Hellwig DH, Brown DC, Dvorak RA. Effect of mannan oligosaccharide (Bio-Mos") supplementation with and without zinc oxide on performance and immunocompetence of weanling pigs [abstract]. J Anim Sci. 2000;78(Suppl 2):61. Abstract 166.
*22. O'Quinn PR, Bergstrom JR, Nelssen JL, Tokach MD, Dritz SS, Goodband RD, Maxwell CJ, Civis CA, Loughmiller JA. The interactive effects among diet complexity, zinc oxide, and feed grade antibiotic on performance of segregated early-weaned pigs. Kansas Agr Exp Stat Progr Rep 795. 1997;795:52-56.
23. Harper AF, Estienne MJ. Efficacy of three potential alternatives to antimicrobial feed additives for weanling pigs. Prof Anim Sci. 2002;18:343-350.
*24. van Eys J, Den Hartog L. Separation of health, performance roles of probiotics may lead to understanding of mode of action. Feedstuffs. May 5, 2003:24-28.
25. McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Inf Dis. 2002;34(suppl 3):S93-S106.
*26. Rozeboom DW, Shaw DT, Pettigrew JE, Connolly A. Comparative effects of mannanoligosaccharide and an antibiotic in nursery diets on performance of pigs reared on three different farms [abstract]. J Anim Sci. 2001;79(suppl 2):79. Abstract 211.
*27. Straw B. Selection and use of antibiotics for swine. George Young Conf Proc; 1994:33-54.
28. Hill GM, Mahan DC, Carter SD, Cromwell GL, Ewan RC, Harrold RL, Lewis AJ, Miller PS, Shurson GC, Veum TL. Effect of pharmacological concentrations of zinc oxide with or without the inclusion of an antibacterial agent on nursery pig performance. J Anim Sci. 2001;79:934-941.
* Non-refereed references.
