Original research |
Peer reviewed |
Sow and litter performance following farrowing induction with prostaglandin: Effect of adjunct treatments with dexamethasone or oxytocin
Desempeño de la hembra y su camada después de la inducción del parto con prostaglandinas: Efectos de los tratamientos complementarios con dexametasona u oxitocina
Performance de la truie et la portée suivant la induction de la mise base avec prostaglandine: Effet de traitements complémentaires avec dexamethasone ou oxytocine
Glen Cassar, DVM, PhD; Roy N. Kirkwood, DVM, PhD, Diplomate ECAR; Robert Friendship, DVM, MSc, Diplomate ABVP; Zvonimir Poljak, DVM, MSc
GC, RF, ZP: Department of Population Medicine, University of Guelph, Guelph, Ontario, Canada; RNK: Department of Large Animal Clinical Sciences, Michigan State University, East Lansing, Michigan; Corresponding author: Dr Roy Kirkwood, Department of Large Animal Clinical Sciences, Michigan State University, East Lansing, MI 48824-1314; Tel: 517-432-5198; E-mail: kirkwood@cvm.msu.edu.
Cite as: Cassar G, Kirkwood RN, Friendship R, et al. Sow and litter performance following farrowing induction with prostaglandin: Effect of adjunct treatments with dexamethasone or oxytocin. J Swine Health Prod. 2005;13(2):81-85.
Also available as a PDF.
SummaryObjective: To evaluate effects of dexa-methasone (DEX) and oxytocin in prosta-glandin F2[alpha] (PGF) farrowing-induction protocols. Materials and methods: In Experiment One, 144 sows were induced with two injections of PGF 6 hours apart (split dose) with or without injection of 20 mg DEX with the second PGF. Interval from initial PGF to farrowing, duration of farrowing, litter size born alive, number of stillbirths, and piglet weight gain to 10 days of age were recorded. In Experiment Two, 106 sows were induced with single or split-dose injections of PGF with or without injection of 20 IU oxytocin 24 hours after initial PGF. Time to onset and duration of farrowing were recorded, as were requirement for manual intervention, total litter size born, and incidence of stillbirths. Results: For sows farrowing 24 to 32 hours after initial PGF injection in Experiment One, there was no effect of DEX treatment on the PGF-to-farrowing interval, duration of farrowing, or piglet growth and survival to 10 days of age. In Experiment Two, more sows farrowed by 32 hours after the split dose of PGF than after a single dose (P < .05). The PGF injection protocol did not influence the farrowing response to oxytocin. Oxytocin injection was associated with higher stillbirth rates when cervical dilation was incomplete. Implications: These data do not support a role for corticosteroid in farrowing induction protocols. Oxytocin administered 24 hours after PGF (single or split dose) was associated with farrowing problems, suggesting that routine use of oxytocin in periparturient sows is contraindicated. | ResumenObjetivo: Evaluar los efectos de la dexametasona (DEX por sus siglas en inglés) y de la oxitocina en los protocolos de inducción de parto de la prostaglandina F2[alpha] (PGF por sus siglas en inglés). Materiales y Métodos: En el Experimento Uno, 144 hembras fueron inducidas con dos inyecciones de PGF con 6 horas de separación (dosis dividida) con o sin una inyección de 20 mg de DEX con la segunda dosis de PGF. Se registró el intervalo de PGF al parto, duración del parto, número de nacidos vivos, nacidos muertos y la ganancia de peso del lechón hasta los 10 días después del nacimiento. En el Experimento Dos, 106 hembras fueron inducidas con una inyección única o con dosis divididas de PGF con o sin inyección de 20 UI de oxitocina, 24 horas después de la inyección inicial de PGF. Se registraron el tiempo de inicio del parto su duración, si hubo o no necesidad de asistir el parto, tamaño total de la camada y la incidencia de nacidos muertos. Resultados: En el Experimento Uno, para las hembras que parieron 24 a 32 horas después de la inyección de no hubo efecto del tratamiento de la DEX en el intervalo de aplicación de PGF a parto, duración del parto o crecimiento del lechón y supervivencia a los 10 días de edad. En el Experimento Dos, más hembras (P < .05) parieron 32 horas después de la segunda dosis de PGF que después de la dosis única. El protocolo de la inyección de PGF no influenció la respuesta de parto a la oxitocina. La inyección de oxitocina se asoció a un porcentaje mayor de nacidos muertos cuando la dilatación cervical fue incompleta. Implicaciones: Esta información no apoya el uso de los corticosteroides en los protocolos de inducción de parto. La oxitocina administrada 24 horas después del PGF (dosis única o dividida) se asoció con problemas de parto, sugiriendo que el uso de oxitocina en hembras periparturientas está contraindicado. | ResuméObjectif: Évaluer des effets de la dexamethasone (DEX par ses initiales en anglais) et oxytocin dans les protocoles de induction de mise bas avec prostaglandine F2[alpha] (PGF par ses initiales en anglais). Matériels et méthodes: Dans le Expérience Un, 144 truies ont été induites avec deux injections de PGF, 6 heures à part (dose fendue) avec ou sans injection de 20 mg de DEX avec la deuxième dose de PGF. L'intervalle de PGF à mise bas, durée de la mise bas, nombre de nés vivants, mort-né, et gain du poids du porcelet à 10 jours d'âge ont été enregistrés. Dans le Expérience Deux, 106 truies ont été induites avec un injection unique ou de une dose fendue de PGF avec ou sans injection de 20 UI d'oxytocine 24 heures après le PGF initial. Le temps à début et durée de mise bas a été enregistré, même que étaient le besoin pour intervention manuelle, nombres de nés totaux, et mort-nés. Résultats: Dans la Expérience Un, pour les truies accouchant 24 à 32 heures après l'injection initiale de PGF, il n'y avait aucun effet de traitement avec la DEX sur l'intervalle de application de PGF- à -accouchement, durée d'accouchement, ou augmentation du poids des porcelets et survie à 10 jours d'âge. Dans la Expérience Deux, plus de truies (P < .05) accouché par 32 heures après la dose fendue de PGF qu'après une dose unique. Le protocole d'injection de PGF n'a pas influencé la réponse du accouchement à l'oxytocin. L'injection d'oxytocin a été associée avec plus hauts taux de porcelets mort-nés quand la dilatation cervicale était incomplète. Implications: Ces données ne supportent pas le rôle du corticosteroid dans les protocoles de l'induction de la mise bas. L'oxytocine administré 24 heures après la PGF (unique ou dose fendue) été associé avec les problèmes d'accouchement, en suggérant que l'usage d'oxytocin dans les truies periparturient est contre-indiqué. |
Keywords: swine, farrowing,
prostaglandin F2[alpha], PGF, dexamethasone, DEX, oxytocin
Search the AASV web site
for pages with similar keywords.
Received: March
4, 2004
Accepted: September
14, 2004
In addition to initiating piglet delivery, peripartum endocrine changes may also affect early postnatal piglet survival. High levels of maternal corticosteroids are involved in advancing fetal visceral (ie, lung and intestinal) maturation, which may impact postnatal survival.1,2 Further, the prepartum injection of 100 mg of prednisolone has been associated with a reduced duration of farrowing and increased piglet survival to 3 days of age.3 The effect on piglet survival must be interpreted with caution, since any direct effect of cortico-steroid is confounded with effects on duration of farrowing, although enhanced piglet neonatal growth has been observed following a prepartum injection of 20 mg dexameth-asone.4
The objective of induced farrowing is to allow increased supervision of piglet delivery to improve neonatal survival.5 To induce parturition, manufacturers recommend that a single intramuscular injection of prostaglandin F2[alpha] (PGF) or PGF analogue be administered up to 2 days before due date. This protocol usually results in approximately 50% to 60% of sows farrowing the next working day.6 However, the farrowing response was markedly improved when two injections of PGF were administered 6 hours apart (split-dose protocol).7 Although the predictability of the day of farrowing was improved by the split-dose induction protocol, the time during the day at which the sow farrowed remained variable.
To improve the synchronization of farrowing, some producers inject oxytocin 20 to 24 hours after a single PGF injection, which usually results in a more rapid delivery of the first pig. However, this use of oxytocin also often increases the need for manual intervention, because it is associated with a higher incidence of interrupted farrowings.8,9 An interrupted farrowing is characterized by a prolonged interval between delivery of the first pig and the subsequent piglets. Why some sows experience an interrupted farrowing is not known. However, it may be that the injection of oxytocin occurs before complete cervical dilation and so results in a painful delivery of the first piglet. In turn, pain may induce release of epinephrine and result in a transient tocolysis. When oxytocin was administered after delivery of the first piglet and so, presumably, after complete cervical dilation, a shorter duration of farrowing resulted, with no evidence of interrupted farrowings.10 From the above, we reasoned that the improved farrowing response to a split dose of PGF suggests that more sows will have complete cervical dilation at 24 hours after PGF injection and so may be less likely to experience farrowing problems associated with oxytocin treatment.
The objectives of the present experiments were to further examine the effect of dexamethasone on the farrowing response of the sow and growth of the litter, as well as to determine the incidence of oxytocin-associated farrowing problems after induction with a single or split dose of PGF.
Materials and methods
Animals and facilities
These studies were approved by the animal care committees of the University of Guelph and Michigan State University and were conducted in accordance with their guidelines for the care and use of experimental animals. Experiment One was conducted on each of two facilities, one a commercial 700-sow farrow-to-feeder facility in Guelph, Ontario, Canada, and the other a 220-sow farrow-to-finish facility at Michigan State University. Experiment Two was conducted at the Guelph facility.
Experimental design
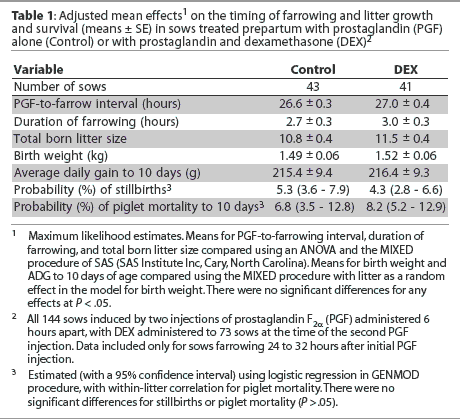
For Experiment One, 144 mixed-parity sows were induced to farrow 2 days before their due date (day 113 of gestation) with two vulvar injections of 2.5 mg or 5.0 mg prostaglandin F2[alpha] (PGF; Lutalyse, Pharmacia, Orangeville, Ontario) administered 6 hours apart by 12-mm, 20-gauge needle. The different dosages reflect different management protocols for each farm but, on the basis of previous data,6 no dose-dependant difference in farrowing response was anticipated. The initial injection was administered between 7:00 am and 8:00 am. At the time of the second injection, sows were assigned to receive an injection of 20 mg dexamethasone (DEX; Dexadreson, Intervet Canada, Whitby, Ontario; n = 73) or to serve as controls (n = 71). This dose of dexamethasone is at the high end of the therapeutic range and was administered intramuscularly (IM) in the neck.
The following working day (24 to 32 hours after initial PGF injection), sows were monitored continuously for piglet delivery until farrowing was complete. If an interval between piglet deliveries exceeded 45 minutes, manual intervention was employed. Sows farrowing < 24 hours after initial PGF injection were not observed, and their data were not included in the analysis. Similarly, sows farrowing > 32 hours after initial PGF injection were deemed to be nonresponsive to the induction protocol and excluded from data analysis. Piglets of sows farrowing 24 to 32 hours after PGF injection were individually identified by ear notching at birth, and incidences of piglet mortality were recorded. For sows farrowing 24 to 32 hours after initial PGF injection, records were maintained for the interval from initial PGF injection to onset of farrowing, duration of farrowing, litter size born (alive and stillborn), and piglet weights and survival at birth and at 3 and 10 days of age.
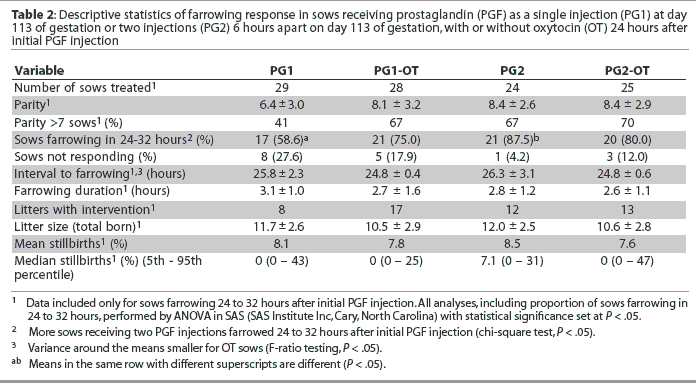
For Experiment Two, 106 mixed-parity sows were assigned, 2 days before their due-to-farrow date, to injection of 5 mg PGF (PG1; n = 29); injection of 5 mg PGF followed 24 hours later by 20 IU oxytocin (Bimeda-MTC Pharmaceuticals, Cambridge, Ontario) (PG1-OT; n = 28); injection of 2.5 mg PGF followed in 6 hours by a second injection of 2.5 mg PGF (PG2; n = 24); or injection of 2.5 mg PGF followed in 6 hours by a second injection of 2.5 mg PGF and then 20 IU oxytocin 24 hours after the initial PGF injection (PG2-OT; n = 25).
The dose of oxytocin was based on literature evidence indicating effective doses of between 10 and 30 IU8,9,11 and anecdotal evidence of 20 IU being a commonly used dose in commercial practice. The PGF was administered into the vulva and the oxytocin was administered IM in the neck. Initial PGF injections were administered between 7:00 am and 8:00 am on day 113 of gestation. During the following working day, sows were monitored continuously for piglet delivery until farrowing was complete. If an interval between piglet deliveries exceeded 45 minutes, manual intervention was employed. As in Experiment One, sows farrowing < 24 hours or > 32 hours after initial PGF injection were excluded from data analysis. Where oxytocin injection was indicated, an assessment of cervical dilation was performed prior to injection. A gloved hand was inserted into the vagina and cervical dilation confirmed if at least two fingers could be inserted comfortably into the cervical canal. Records were maintained for interval from initial PGF injection to onset of farrowing, duration of farrowing, requirement for manual intervention, and litter size born (alive and stillborn).
Statistical analysis
All analyses were performed by ANOVA using SAS (SAS Institute Inc, Cary, North Carolina). The treatment means for intervals from initial PGF injection to delivery of the first pig, duration of piglet delivery, and total born litter size were compared using the MIXED procedure. The proportion of sows farrowing 24 to 32 hours after initial PGF injection and proportion of stillbirths were analysed using logistic regression in GENMOD procedure and tested by the Wald chi-square test. Differences in the variances around the means were tested by F-ratio test and analyses were adjusted for parity. Data from Experiment Two were analyzed as a 2 x 2 factorial.
For Experiment One, treatment effects on piglet birth weights and average daily gain to 10 days of age were compared for all piglets using the MIXED procedure with litter as a random effect and piglet birth weight as covariate in the model for average daily gain. Treatment effects on mortality of piglets until 10 days of age were tested using logistic regression in GENMOD procedure with birth weight as covariate and accounting for the within-litter correlation using compound symmetry correlation structure. Separate analyses for average daily gain and mortality were performed for all piglets with birth weight < 1.1 kg.
Results
In Experiment One, 24 sows from each treatment farrowed < 24 hours after initial PGF injection and were not included in data analysis. Of the remaining sows, 41 DEX sows and 43 control sows commenced farrowing 24 to 32 hours after the initial PGF injection, and the response was not affected by DEX treatment. Eight DEX and four control sows farrowed > 32 hours after initial PGF injection and were not included in the data analysis. Manual intervention was performed in eight sows per treatment. For sows farrowing 24 to 32 hours after initial PGF injection, there was no effect of DEX on the PGF-to-farrowing interval, the duration of the farrowing process, total born litter size, stillbirth rate, or piglet growth or mortality to 10 days of age (Table 1). Similarly, there were no effects of DEX when analyses were restricted to piglets with birth weights < 1.1 kg.
In Experiment Two, more sows receiving two PGF injections farrowed 24 to 32 hours after initial PGF injection than did those receiving a single PGF injection (P < .05; Table 2). There was no effect of oxytocin treatment on numbers of sows farrowing 24 to 32 hours after PGF injection. However, for sows farrowing 24 to 32 hours after the first PGF injection, the variance in the interval to farrowing was less (P < .05) for oxytocin-treated sows (Table 2). In sows that responded to induction, there was no overall treatment effect on the PGF-to-farrowing interval, farrowing duration, percent live births, or the need for manual interventions (Table 2). For the eight oxytocin-treated sows that farrowed > 32 hours after initial PGF injection, the stillbirth rate was 50%, while the stillbirth rate was 14% for the nine sows farrowing > 32 hours after initial PGF injection that did not receive oxytocin.
Discussion
The data presented for Experiment One indicate no effect of dexamethasone on the timing or duration of farrowing. An earlier report had shown that prepartum injection of prednisolone resulted in a shorter period of piglet delivery.3 An explanation for the shorter delivery time was not provided, but it is reasonable to infer the involvement of an analgesic effect of the corticosteroid allowing for a more comfortable delivery. However, the sows used in the present study were relatively mature and so less likely to suffer a painful delivery. If true, an effect of dexamethasone on the piglet delivery process may become apparent only in young sows.
Other authors have demonstrated that dexamethasone treatment of the periparturient sow resulted in enhanced neonatal piglet growth, especially of the low-birth-weight pigs.4 Also, injection of dexamethasone into newborn piglets may improve growth, although the effect has proven inconsistent with either a general or a sex-linked growth response, or no growth response being observed.12-14 In the present study, no effect of dexamethasone was observed on litter growth and survival regardless of birth weight. Therefore, given the unpredictable response to corticosteroid treatment, the use of dexamethasone in the farrowing induction protocol does not appear to be warranted.
In Experiment Two, the farrowing response to induction supports previous reports that the vulval route for PGF injection produces acceptable results at lower than label dosages.6,15 Further, in terms of the numbers of sows farrowing 24 to 32 hours after initial PGF injection, the split-dose PGF protocol produced a superior response compared to the single dose, also supporting earlier observations.7 In the present study, oxytocin did not result in a general increase of stillbirths in sows with a dilated cervix, but may have been a factor in the farrowing complications and the high number of stillbirths in sows with a closed cervix at the time of oxytocin treatment. An increased stillbirth rate associated with the use of oxytocin has been observed previously.16 Since evaluation of cervical dilation is not routinely performed prior to oxytocin injection, the prepartum use of oxytocin cannot be recommended.
Recent research has suggested that even after delivery of the first piglet, the use of oxytocin might produce undesirable results.10 The latter authors described larger numbers of stillbirths per litter, with the highest incidence being among the first four pigs rather than towards the end of farrowing. This pattern of stillbirth deliveries was associated with an increased incidence of umbilical cord abnormalities, suggesting that inappropriately powerful uterine contractions may be detrimental to piglet survival even when the cervix is fully patent. In the present study, oxytocin-treated sows had numerically more farrowing problems requiring manual intervention (30 compared to 20 not requiring intervention), but there were too few sows to detect a significant difference. This research would suggest that there is little to be gained by routine use of oxytocin as part of an induction program. Indeed, the potential for oxytocin to cause problems if dilation of the cervix has not advanced sufficiently to allow easy passage of the piglets, and its potential to cause interrupted farrowings, suggest that the routine use of oxytocin in periparturient sows is contraindicated.
Implications
- Administration of dexamethasone to periparturient sows does not impact neonatal piglet growth or survival.
- The use of a split-dose PGF induction protocol decreases the likelihood of sows not farrowing in response to PGF.
- As some sows may have a nondilated cervix 24 hours after initial PGF injection, even with split-dose PGF induction, the use of oxytocin in periparturient sows is contraindicated.
Acknowledgment
This work was generously supported by Ontario Pork.
References
1. Olson EB Jr. Role of glucocorticoids in lung maturation. J Anim Sci. 1979;49:225-238.
2. Moog F. Endocrine influences on the functional differentiation of the small intestine. J Anim Sci. 1979;49:239-249.
*3. Bilkei G. The effect of prednisolon on perinatal mortality of piglets. Proc 12th Congress IPVS, The Hague, The Netherlands. 1992;2:465.
4. De Rensis F, Sottocorona M, Kirkwood RN. Effect of prostaglandin and dexamethasone injection on farrowing and piglet neonatal growth. Vet Rec. 2002;151:330-331.
5. Holyoake PK, Dial GD, Trigg T, King VL. Reducing pig mortality through supervision during the perinatal period. J Anim Sci. 1995;73:3543-3551.
6. Kirkwood RN, Thacker PA, Aherne FX, Goonewardene LA. The effect of dose and route of administration of prostaglandin F2a on the parturient response of sows. Swine Health Prod. 1996;4:123-126.
7. Kirkwood RN, Aherne FX. Increasing predictability of cloprostenol-induced farrowing in sows. Swine Health Prod. 1998;6:57-59.
8. Welp C, Jochle W, Holtz W. Induction of parturition in swine with a prostaglandin analog and oxytocin: A trial involving dose of oxytocin and parity. Theriogenology. 1984;22:509-520.
9. Kirkwood RN, Thacker PA. Effect of propranolol on the onset and duration of parturition in sows. Can Vet J. 1995;36:238-239.
10. Mota-Rojas D, Martinez-Burnes J, Trujillo-Ortega ME, Alonso-Spilsbury ML, Ramirez-Necoechea R, Lopez A. Effect of oxytocin treatment in sows on umbilical cord morphology, meconium staining, and neonatal mortality of piglets. Am J Vet Res. 2002;63:1571-1574.
11. Dial GD, Almond GW, Hilley HD, Repasky RR, Hagen JC. Oxytocin precipitation of prostaglandin-induced farrowing in swine: determination of the optimal dose of oxytocin and optimal interval between prostaglandin F2 alpha and oxytocin. Am J Vet Res. 1987;48:966-970.
12. Carroll JA. Dexamethasone treatment at birth enhances neonatal growth in swine. Dom Anim Endocrinol. 2001;21:97-109.
13. Gaines AM, Carroll JA, Allee GL, Yi GF. Pre- and postweaning performance of pigs injected with dexamethasone at birth. J Anim Sci. 2002;80:2255-2262.
*14. Young MG, Tokach MD, Dritz SS, Goodband RD, Nelssen JL. Effect of dexamethasone injection at birth on growth performance of pigs from birth to weaning. Kansas State University Swine Day. 2002;20-22. Available at http://www.oznet. ksu.edu/library/lvstk2/samplers/srp897.asp. Accessed January 7, 2005.
15. Friendship RM, Templeton CL, Deckert AE. An evaluation of vulvomucosal injections of prostaglandins for induction of parturition in swine. Can Vet J. 1990;31:433-436.
16. Yang P-C, Fang W-D, Huang S-Y, Chung W-B, Hsu WH. Farrowing induction with a combination of prostaglandin F2[alpha] and a peripherally acting [alpha]2-adrenergic agonist AGN 190851 and a combination of prostaglandin F2[alpha] and oxytocin. Theriogenology. 1996;46:1289-1293.
* Non-refereed references
