Commentary |
Non refereed |
The porcine reproductive and respiratory syndrome quandary. Part II: Vaccines and vaccination strategy
William L. Mengeling, DVM, PhD, Diplomate ACVM
Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa
Cite as: Mengeling WL. The porcine reproductive and respiratory syndrome quandary. Part II: Vaccines and vaccination strategy. J Swine Health Prod. 2005;13(3):152-156.
Also available as a PDF.
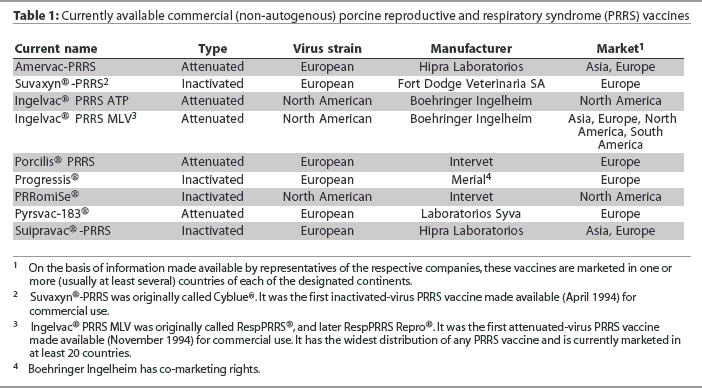
The first vaccine to aid in the prevention of the porcine reproductive and respiratory syndrome (PRRS) was registered for use in Spain in 1994. It comprised an inactivated European strain of PRRS virus (PRRSV) that had been propagated in porcine alveolar macrophages. Later in the same year, the first attenuated-virus vaccine became commercially available in the United States.1 It comprised a North American strain of PRRSV that had been propagated in an established monkey kidney cell line. Since 1994, a number of additional vaccines (comprising either attenuated or inactivated PRRSV) have been developed in both the United States and Europe. Each includes a different strain of the virus of either the North American or European genotype (Table 1).
Although PRRS vaccines have been used extensively over the past decade, there have been recurring questions in regard to their efficacy, and, in the case of attenuated-virus vaccines, their safety. As a consequence, a great deal of time, effort, and financial resources have been directly or indirectly devoted to a better understanding of PRRS immunology and the development of what has sometimes been optimistically referred to as the next generation of PRRS vaccines. Just how this might be accomplished is as yet unclear, and other than the still controversial issue of genotype specificity,2,3 there is no definitive evidence that the origina;l vaccines are less effective than any of those developed more recently. But despite disappointments of the past, research on PRRS immunology, including vaccine development, is continuing, perhaps even being accelerated, largely because of the major economic impact of the disease4 and the recent increase in research funding.5 To provide some indication of the probability of soon developing better approaches for PRRS immunoprophylaxis, the following discussion briefly addresses some of the advantages and disadvantages of the types of vaccine that are most likely to be considered and a possible strategy to increase the immune response to vaccines currently available.
Virulent virus
Although virulent virus does not meet the definition of a vaccine, it is included here, because there seems to be a growing tendency in the United States to use virulent strains of PRRSV in an attempt to induce immunity for the reproductive facet of the syndrome.6 In general, the most common approach - often referred to as planned or controlled exposure - is as follows. Females are exposed, before conception and typically during the acclimatization phase of gilt development, to the predominant or only strain of field virus circulating in the herd at the time. Expectations are that any clinical reaction to the virus will be minimal, or at least manageable, and the associated immune response will be directed to the strain of PRRSV to which the females are most likely to be at risk during their subsequent gestation(s). With the exception of a possible untoward clinical reaction during the immunization process, it is difficult to imagine a more effective way of inducing protective immunity. However, the potentially negative consequences of purposely infecting females (destined to join the breeding herd) with fully virulent PRRSV are likely to deter most veterinarians and producers from considering this approach. Moreover, planned exposure may not always result in the theorized level of immunity. Notably in two herds that have come to my attention, reproductive failure was a consequence of purposeful exposure of pregnant females to the same strain of field virus previously used for immunization. Whether it was less severe than it might have been if a commercially available attenuated-virus vaccine had been used for immunization prior to exposure to virulent virus during gestation is impossible to evaluate. Additional details in regard to these two herds, as well as a herd that was naturally exposed to what was believed to be the same strain of virulent virus in each of two succeeding gestations, have been presented elsewhere.7 But regardless of the level of immunity provided by exposure to virulent virus before conception, a clear downside is the certainty of maintaining such virus in the herd in perpetuity.
Attenuated-virus (modified-live-virus) vaccine
Of all of the types of conventional vaccines available today, those prepared from attenuated virus are the most likely to provide the highest level of clinical protection. They have the potential to replicate extensively over a long period of time in the vaccinated pig (the degree of replication may depend on the level of attenuation) and thus repeatedly expose the pig's immune system to the entire spectrum of viral antigens. Because neutralizing antibody against PRRSV (ie, antibody that probably plays an important role in protective immunity as well as in clearing the virus from a pig) is slow to develop, this long interval of replication may be more important for PRRS immunity than it is for many other viruses. However, despite the fact that it is probably the best vaccine of the group in regard to protection, it still falls short of expectations in some instances. At one time the biggest criticism of attenuated virus was its potential to revert to some degree of virulence if it were used under conditions that allowed its sequential passage in naive pigs. This concern for safety is apparently of less concern today, in that even fully virulent virus is sometimes being administered to gilts in an attempt to induce immunity before conception. And keep in mind that while there are reasonable concerns about the efficacy of attenuated-virus vaccines under some field conditions, controlled studies have confirmed their appreciable protective value when they are administered well before exposure to virulent virus, especially in regard to the respiratory facet of the disease.8,9
Deletion-mutant-virus vaccine
Assuming that the immunogenic and replication properties of the virus are not markedly altered by deletion of some part(s) of the viral genome, deletion-mutant-virus vaccine should have the ability to stimulate a protective immune response similar to that of attenuated-virus vaccine. And on the plus side, the deletion(s) could result in either or both of two properties that would give deletion-mutant-live-virus vaccine an advantage over conventional live-virus vaccines. First, the deletion mutant might be unable to regain any degree of virulence by reverse mutation (although it might still do so as a consequence of recombination). In fact it might actually be attenuated by the deletion(s). Second, with the development of a companion diagnostic test (directed toward the antigen or epitope coded for by the deleted portion of the viral genome), it might be possible to identify pigs exposed to virulent virus regardless of vaccination history. Of course, the latter is what is most often thought of as the salient feature of deletion-mutant-virus vaccines. Because virulent PRRSV can persist in an infected pig for a long period of time, perhaps even in a previously vaccinated pig, this attribute might be particularly important should an eradication program be started (assuming that vaccination with live virus would still be allowed during the program). But also be aware that there is no reason to believe that a deletion-mutant-live-virus vaccine would be any more effective in regard to immunity than would a non-deleted virus, and it is conceivable that it would be less so.
The bad news is that it may not be possible, or it may be at the very least very difficult, to develop a suitable deletion-mutant-live-virus vaccine because of the nature of PRRSV genome. Namely, the genome is a relatively short, single strand of RNA, and, in addition, all of the genes that code for structural proteins may be essential for replication.10,11 To date (to my knowledge), most deletions have resulted in lethal mutants (ie, the altered genome will no longer replicate infectious virus). This is in stark contrast to the relative ease with which deletion mutants have been created with large double-stranded DNA viruses like pseudorabies (Aujeszky's disease) virus. And perhaps the notable success with pseudorabies virus in both the development of deletion-mutant-live-virus vaccines and the use of such vaccines in eradication programs has resulted in unrealistic expectations for PRRSV.
There is also a double challenge faced in the practical application of a deletion-mutant-virus vaccine in an eradication program. First, the deleted portion of the viral genome must be present in all virulent field strains of PRRSV. Otherwise, of course, some virulent strains would mimic the vaccine in regard to the missing "diagnostic antigen." Second, the immune response to the diagnostic antigen (which could be all or part of a distinct protein, or simply a single epitope) must always be detectible via the companion diagnostic test. For example, in the case of pseudorabies, it was shown - fortunately early in the eradication program - that one of the proposed differential diagnostic tests was not sufficiently sensitive.12 The reason was that not all infected pigs produced an adequate level of antibody to test positive, even though the antigen was present in all field strains. This observation, although seldom mentioned, was an important contribution to the success of the pseudorabies eradication program in the United States.
Inactivated-virus vaccine
In general, inactivated vaccines are used when the level of immunity they provide, which is typically less, often considerably less, than provided by live-virus vaccines, is judged to be "good enough." Notable examples are swine influenza virus and porcine parvovirus vaccines. Unfortunately, the efficacy of inactivated PRRS vaccines has been seriously questioned. Because the issue of inactivated PRRS vaccine and efficacy has been debated so many times, often passionately, in so many forums, I won't pursue it further in this context. Other real or potential disadvantages of inactivated-PRRSV vaccine are cost and the need in most cases for more than a single dose to stimulate a measurable antibody response.
The biggest advantage of an inactivated-PRRSV vaccine is that of almost unquestioned safety, both real and perceived. What I mean by the latter is that if there is any clinical problem, short of the ridiculous, after the administration of live-virus vaccine, it is logical to at least consider the vaccine as the possible culprit. The associated stress for the swine producer, the veterinarian, and the vaccine producer can largely be circumvented by using inactivated vaccine. That is, it is likely that an inactivated vaccine will be considered innocent until proven otherwise, whereas it is just as likely that an attenuated-virus vaccine will be considered guilty until proven otherwise. On the other hand, from an ethical perspective, it is important to be assured that a particular vaccine is efficacious or the issue of safety is a moot point.
Inactivated-virus vaccine can also be prepared from a deletion-mutant virus (either attenuated or virulent if available) or deleted in some other manner so that vaccination does not obscure detection of pigs exposed to virulent live virus. The principle and limitations would be the same as those described above for deletion-mutant-live-virus vaccines.
Naked DNA vaccine
Our very limited experience with a naked-DNA-PRRS vaccine (while I was still on the staff of the National Animal Disease Center) confirmed that it could be used to raise antibody against the virus. However, there was no evidence (among the few pigs tested) that it provided any clinical protection. I believe, although I am not sure, that the same result was obtained by a large biologics company. It is a safe vaccine from the standpoint that it can be constructed so that it does not replicate infectious virus. In fact, the DNA vaccine we tested coded for only a single PRRSV protein, namely, the envelope protein that is thought to be the most important PRRSV protein for raising neutralizing antibody. Proteins (antigens), by virtue of being produced within the DNA-transfected (infected) cell, are presented to the immune system in the same way as are those produced in live-virus-infected cells, which in turn is a route of presentation that is reported to effectively stimulate the cell-mediated arm as well as the humoral arm of the immune system (in contrast to inactivated virus vaccines and subunit vaccines that are thought to primarily stimulate the humoral arm of the immune system). One general concern that has been expressed relative to DNA vaccines, especially in regard to people, is the possibility that the DNA present in the vaccine might incorporate into the cell genome of the vaccine recipient, with some as yet undefined, but possibly undesirable, result.
Protein subunit vaccine
What was said above about inactivated-virus vaccines essentially applies to protein-subunit vaccines. In general, only the means by which they are prepared differ. That is, inactivated-virus vaccine is, as the name implies, whole virus that is disabled, usually by some chemical treatment, so that it no longer has the ability to replicate, whereas subunit-protein vaccine (as defined here) is produced in part through the miracle of genetic engineering. As an example, one or more genes (that code for the corresponding selected proteins) of the PRRSV genome might be inserted into the genome of another virus such as a baculovirus. The baculovirus might then be propagated in cell culture, whereby both baculovirus and PRRSV proteins would be produced. The next step might entail purification of the PRRSV proteins to rid the vaccine of at least most baculovirus and cell proteins.13
Vectored vaccine
In theory, this type of vaccine has a great deal of merit. The idea is that the gene or genes of the most important immunogenic protein or proteins of one or more pathogenic viruses (those for which we want to stimulate immunity) are genetically engineered into the genome of another virus (the vector). The vector does not have to be a virus, for example it could be a bacterium, but I will focus on a virus for this short discussion. When a person or a lower animal such as a pig is injected with the genetically altered vector, the vector replicates and not only codes for its own proteins but also for proteins corresponding to the inserted gene(s). Assuming that genetic engineering can be accomplished satisfactorily, the concept seems very promising. However, there are a few hurdles, some of which present a major problem and most of which involve the selection of suitable vector.
First, the vector must be large enough to accommodate the inserted gene(s). Prime candidates are large double-stranded DNA viruses such as the following that are listed in the order of their increasing capacity: adenoviruses, herpes viruses (eg, pseudorabies virus), and poxviruses (eg, vaccinia virus and swinepox virus).
Second, the vector should probably not be a natural pathogen of pigs, otherwise pigs might already have antibody to the vector, which in turn would have the potential to interfere with the necessary replication of the vector. Obviously, preexisting antibody to a vector would have nothing to do with preexisting antibody - and by inference preexisting immunity - to the virus or viruses for which the vaccine was intended. This is an important point that I will emphasize in yet another way. If we try to vaccinate for a particular pathogen using a conventional vaccine and the vaccine is ineffective because there is preexisting antibody, either passively or actively acquired, we can assume that the vaccine recipient is already protected, albeit only temporarily in the case of passively acquired antibody. On the other hand, if the vaccine is ineffective because of preexisting antibody against a vector, we may be left with a completely susceptible individual in regard to the pathogens for which the vaccine was intended. On the potentially positive side of all this is the possibility that if the vaccinated pig had antibody, eg, passively acquired antibody, against the virus or viruses to which immunity is desired, but no antibody against the vector, the vector, unscathed, plus its load of inserted genes, might reach cells of the immune system; and once inside such cells, nascent proteins would, at least in theory, be unaffected by circulating antibody.14 The vector would thus serve as a "Trojan horse." Unfortunately, the practice may not fully parallel the theory. The possible reasons will not be discussed here.
Third, if we identify a potential vector for which the pig will be unlikely to have preexisting antibody, we must be sure that the same vector will replicate extensively in the pig so that there will be adequate production of the coded proteins, ie, those corresponding to the vectored genes.
Fourth, the vector should be incapable of infecting another species that might come in contact with vaccinated pigs, especially if infection of another species results in clinical disease. This issue may be even more restrictive if the vector has the potential to infect people (eg, vaccinia virus), because then the definition of a potential problem is likely to be expanded to include those who might be immunocompromised by a disease such as AIDS, or a treatment such as chemotherapy. Imagine the complexities associated with trying to determine if there would ever be a problem under such conditions. So while the concept of a vectored vaccine for PRRSV is appealing, the practicalities are somewhat daunting.
Potential for the combined use of vaccines
There is a possibility that sequential administration of more than one type of PRRS vaccine will result in a level of protective immunity appreciably greater than that following the administration of a single type of vaccine or a single exposure to virulent virus. Moreover, the combination may be more effective regardless of how many times either virulent virus or a single type of vaccine is administered alone, ie, the same strain and virulence level administered repeatedly.
Such a combination strategy, referred to previously as the SWINE method of PRRS (or pathogen) control, was presented recently, along with supporting data, at the annual meeting of the American Association of Swine Veterinarians in Toronto.7 Its purpose is to provide protection against maternal reproductive failure via actively acquired immunity, as well as clinical disease in young pigs via passively acquired immunity. In addition to the amelioration of clinical disease, it has the potential to markedly reduce shedding of virulent virus. Its identifying acronym, namely SWINE, is derived from the five discrete phases of the strategy (referred to here as steps for simplicity): "Sensitization," "Wait," "Immunization," "Neutralization," and "Eradication."
Step 1 is sensitization of the immune system with either inactivated virus or attenuated virus beginning when pigs (replacement gilts or boars) are only a few weeks of age. Step 2 is to wait at least several months for appreciable maturation of the immune system, particularly for the relevant protective antigens. Step 3 is to expose sensitized (primed) gilts (or boars) to virulent virus if previous sensitization was with either inactivated virus or attenuated virus, or with attenuated virus if previous sensitization was with inactivated virus. Assuming that the latter (ie, inactivated virus for sensitization and attenuated virus for immunization) would provide an acceptable level of protective immunity, this would clearly be the strategy of choice. It would avoid the periodic reintroduction of virulent virus into the herd as well as minimize the potential for shedding of attenuated virus. And please be aware that mention of virulent virus in this context is not to be construed as a recommendation for its use, but merely as a statement of likely immune responsiveness. Also notice in regard to the above that sensitization and immunization are defined as such relative to their roles in the SWINE method of PRRS (pathogen) control; immunization is always with a form of the virus that presumably has a greater propensity for immune stimulation than what was used for sensitization; and "(pathogen)" is added to the SWINE method of PRRS control because there is the likelihood that the sensitization-wait-immunization steps can be used simultaneously for any of several other pathogens that commonly plague the swine industry. An important point in considering the use of inactivated vaccine for sensitization (a possibility not yet adequately tested under controlled conditions) is that there must be a clear indication of sensitization. If after administering an inactivated product several times there is no obvious, measurable evidence of an immune response, it is unlikely that the immune system can be considered adequately sensitized. Step 4, neutralization, would logically follow success of Steps 1, 2, and 3. And the definition of neutralization is in the broad sense referring to prevention of infection regardless of the means by which it is accomplished by the immune response. Step 5, eradication, would be a realistic goal once the incidence of infection was markedly reduced.
Unfortunately, there appear to be at least two major challenges to any near-term, industry-wide implementation of the SWINE method of PRRS (pathogen) control. First is the question of whether genetic suppliers can provide the estimated 2,500,000 naive gilts (both antibody-free and virus-free) needed to annually repopulate commercial breeding herds - namely the candidates for Step 1. And except for the unlikely possibility of stimulating (sensitizing) the immune system in the presence of passively acquired antibody, Step 1 would therefore be limited to only some of the gilts needed each year. Second, assuming that inactivated vaccine is selected for sensitization, there may be a need to develop a commercial product that will adequately and consistently sensitize the immune system. It is as yet unclear whether currently available inactivated vaccines could fulfill this role. And although attenuated virus can be used for sensitization, perhaps even in the presence of a low level of passive acquired antibody, its use would not be a realistic possibility unless pigs (from a PRRSV-free herd) were moved to yet another isolated site for vaccination, with the assurance that they would not again be exposed to PRRSV until they were shipped months later to destination breeding herds for immunization, ie, Step 3. Moreover, managers of the destination herds would have to have acquiesced to the idea of using virulent virus for immunization, a procedure not likely to be widely accepted.
Summary
It is likely that use of conventional attenuated-virus vaccines will continue to be one of our best tools for the prevention and control of PRRS. Whether any new approaches as to how and when vaccines are administered can enhance their effectiveness, within the constraints of today's commercial swine production, remains to be determined. However, it is conceivable that at least the principles of the SWINE method of PRRS (pathogen) control will play an important role in future strategies to reduce the economic impact of PRRS and other infectious diseases that currently plague the swine industry.
Acknowledgements
I thank Drs Alex Eggen, Joan Plana Duran, John Kolb, Arlen Wonderlich, Eric Vaughn, Cinta Prieto, Nils Henke, and Paolo Martelli for their assistance in gathering information used in the development of Table 1.
Some of the information presented in this commentary was previously published in Polish,15 Spanish,16 or both.
References
*1. Gorcyca DE, Schlesinger KJ, Geeding PW, Chladek DW, Short JA. RespPRRS: A new tool for the prevention and control of PRRS in pigs. Proc AASV. Omaha, Nebraska. 1995:1-22.
2. Labarque G, Van Gucht S, Van Reeth K, Nauwynck H, Pensaert M. Respiratory tract protection upon challenge of pigs vaccinated with attenuated porcine reproductive and respiratory syndrome virus vaccines. Vet Microbiol. 2003;95:187-197.
*3. Kovacs F, Schagemann G. Efficacy of Ingelvac" PRRS MLV against European isolates. Proc Int Sym Emerg Re-emerg Pig Dis. 2003:111-112.
*4. Johnson C, Kliebenstein J, Mabry J, Neumann E. The impact of PRRS on the cost of pig production. Proc Allen D. Leman Swine Conf. 2004(suppl):11.
*5. Neumann E. The PRRS initiative: game, set, match. Proc AASV. Des Moines, Iowa. 2004: 381-385.
*6. Hill HT, Mulford JA, Kaisand JJ, Holtcamp A, Reyes C. PRRS control: To hell and back. Proc AASV. Des Moines, Iowa. 2004:369-375.
*7. Mengeling WL. PRRS vaccinology: Past, present, and future. Proc AASV. Toronto, Canada. 2005:289-304.
8. Mengeling WL, Lager KM, Vorwald AC, Clouser DF. Comparative safety and efficacy of attenuated single-strain and multi-strain vaccines for porcine reproductive and respiratory syndrome. Vet Microbiol. 2003;93:25-38.
9. Opriessnig T, Pallares FJ, Nilubol D, Vincent AL, Thacker EL, Vaughn EM, Roof M, Halbur PG. Genomic homology of ORF 5 gene sequence between modified live vaccines and porcine reproductive and respiratory syndrome virus challenge isolates is not predictive of vaccine efficacy. J Swine Health Prod. In press.
10. Welch SK, Jolie R, Pearce DS, Koertje WD, Fuog E, Shields SL, Yoo D, Calvert JG. Construction and evaluation of genetically engineered replication-defective porcine reproductive and respiratory syndrome virus vaccine candidates. Vet Immunol Immunopathol. 2004;102:277-290.
11. Yoo D, Welch SK, Lee C, Calvert JG. Infectious cDNA clones of porcine reproductive and respiratory syndrome virus and their potential as vaccine vectors. Vet Immunol Immunopathol. 2004;102:143-154.
12. Schmitt BJ, Osorio FA, Stroup WW, Gibbs EP. A comparison of different tests to detect antibodies to pseudorabies glycoproteins gX, gI, and gIII in naturally infected feral pigs. J Vet Diagn Invest. 1991;3:344-345.
13. Kreutz LC, Mengeling WL. Baculovirus expression and immunological detection of the major structural proteins of porcine reproductive and respiratory syndrome virus. Vet Microbiol. 1997;59:1-13.
14. Brockmeier SL, Lager KM, Mengeling WL. Vaccination with recombinant vaccinia virus vaccines expressing glycoprotein genes of pseudorabies virus in the presence of maternal antibody. Vet Microbiol. 1997;58:93-103.
*15. Mengeling WL. The porcine reproductive and respiratory syndrome (PRRS) quandary: Fact versus speculation. Proc Kongress Polskiego Towarzystwa Nauk Weterynaryjnych. Warsaw, Poland. 2004:306-309.
*16. Mengeling WL. The porcine reproductive and respiratory syndrome (PRRS) quandary: Fact versus speculation. Proc SEPOR. 2004:7-19.
* Non-refereed references.
