Original research |
Peer reviewed |
Evaluation of an industry-based sanitation protocol for transport vehicles contaminated with porcine reproductive and respiratory syndrome virus
Evaluación de un protocolo de sanidad basado en las prácticas de la industria para el transporte contaminado con el virus del síndrome respiratorio y reproductivo porcino
Évaluation de protocoles de désinfection pour les véhicules de transport contaminés par le virus du syndrome respiratoire et reproducteur porcin
S. A. Dee, DVM, MS, PhD, Diplomate ACVM; John Deen, DVM, MS, PhD, Diplomate ABVP
Swine Disease Eradication Center, University of Minnesota College of Veterinary Medicine, St Paul, Minnesota. Corresponding author: Dr S. A. Dee, Swine Disease Eradication Center, University of Minnesota College of Veterinary Medicine, Room 385C, 1988 Fitch Avenue, St Paul, MN 55108; Tel: 612-625-4786; Fax: 612-625-1210; E-mail: deexx004@umn.edu
Cite as: Dee SA, Deen J. Evaluation of an industry-based sanitation protocol for transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2006;14(3):126-132.
Also available as a PDF.
SummaryObjective: To test a protocol, using conditions found on commercial swine production units, for sanitation of 1:150 scale models of commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus (PRRSV). Methods: Model trailers were experimentally contaminated with PRRSV MN 30-100 and either pressure-washed with cold water alone (Treatment 1) or washed and then disinfected with modified potassium monopersulfate (Treatment 2), quaternary ammonium chloride (Treatment 3), or a phenolic product (Treatment 4), each applied using a hydrofoamer. In Phase One, the presence of PRRSV RNA was evaluated by polymerase chain reaction (PCR) testing of swabs collected from the trailers' interiors immediately after washing and 30, 60, 90, and 120 minutes post treatment. In Phase Two, the presence of viable PRRSV was evaluated by swine bioassay (injection of supernatants from PCR-positive swabs) and housing pairs of sentinel pigs in treated trailers for 2 hours, beginning 90 minutes post treatment. Results: Swabs were PCR-positive 120 minutes post treatment in 18 of 20 trailers receiving Treatment 1, none of 20 trailers receiving Treatments 2 and 3, and two of 20 trailers (suspect reactions) receiving Treatment 4. Viable virus was detected both by swine bioassay and sentinel pig exposure protocols in trailers receiving Treatment 1, and by swine bioassay alone in trailers receiving Treatment 4. Implications: High-pressure washing of transport trailers, followed by 90 to 120 minutes exposure to either modified potassium monopersulfate or quaternary ammonium chloride disinfectants applied with a hydrofoamer is likely to eliminate residual infectious PRRSV. | ResumenObjetivo: Probar un protocolo, bajo las condiciones presentes en las unidades de producción comerciales de cerdo, para el lavado de modelos, a una escala de 1:150, de los vehículos de transporte comercial contaminados con el virus del síndrome respiratorio y reproductivo porcino (PRRSV por sus siglas en inglés). Métodos: Se contaminaron experimental-mente los modelos de camiones con la cepa de PRRSV MN 30-100 y se lavaron a presión solamente con agua fría (Tratamiento 1) o se lavaron y desinfectaron con mono-persulfato de potasio modificado (Tratamiento 2), cloruro de cuaternarios de amonio (Tratamiento 3), o con un producto a base de fenoles (Tratamiento 4), cada uno se aplicó con un máquina formadora de espuma ("hidroespumador"). En la Fase 1, la presencia del RNA del PRRSV se evaluó con la prueba de PCR de hisopos tomados del interior de los traileres inmediatamente después del lavado y 30, 60, 90, y 120 minutos después del tratamiento. En la Fase 2, la presencia del PRRSV se evaluó con el bioensayo porcino (inyección de los supernadantes de las muestras postivas a PCR) e introduciendo durante 2 horas pares de cerdos centinelas en los camiones tratados, iniciando 90 minutos después del tratamiento. Resultados: Los hisopos fueron PCR positivos 120 minutos después del tratamiento en 18 de 20 camiones que recibieron el Tratamiento 1, ninguno en los 20 camiones que recibieron el Tratamiento 2 y 3, y dos de los 20 camiones (reacción sospechosa) que recibieron el Tratamiento 4. Se detectó virus viable en el bioensayo porcino, así como en los cerdos centinelas de los camiones que recibieron el Tratamiento 1, y sólo con el bioensayo porcino en los camiones que recibieron el Tratamiento 4. Implicaciones: Es probable que el lavado con alta presión de los camiones de transporte, seguido de 90 a 120 minutos de exposición a desinfectantes, a base de monopersulfato de potasio modificado o cloruro de cuaternarios de amonio aplicados con el "hidroespumador," elimine los residuos infecciosos del PRRSV. | ResuméObjectif: Tester différents protocoles, utilisant des conditions retrouvées dans des unités de production porcine commerciales, pour la désinfection de modèles à l'échelle (1:150) de véhicules de transport commercial contaminés avec le virus du syndrome respiratoire et reproducteur porcin (PRRSV). Méthodes: Les modèles de remorques étaient contaminés expérimentalement avec l'isolat PRRSV MN 30-100 et soumis à l'un des traitements suivants: lavage avec jet à pression à l'eau froide seulement (Traitement 1), lavage et désinfection avec soit du monopersulfate de potassium modifié (Traitement 2), soit un chlorure d'ammonium quaternaire (Traitement 3), ou soit un produit phénolé (Traitement 4), chacun appliqué à l'aide d'un appareil moussant. Dans la Phase 1, la présence d'ARN du PRRSV a été évaluée à l'aide d'une épreuve d'amplification en chaîne par la polymérase (PCR) à partir d'écouvillons prélevés de l'intérieur des remorques immédiatement après le lavage ainsi qu'après 30, 60, 90, et 120 minutes suivant le traitement. Dans la Phase 2, la présence de PRRSV viable a été évaluée à l'aide d'un bio-essai utilisant des porcs (injection des surnageants des écouvillons positifs dans l'épreuve PCR) ainsi qu'en hébergeant pendant 2 heures des paires d'animaux sentinelles dans les remorques traitées, 90 minutes suivant le traitement. Résultats: Les écouvillons étaient positifs dans l'épreuve PCR, 120 minutes post-traitement, pour 18 des 20 remorques ayant reçu le Traitement 1, pour aucune des remorques recevant les Traitements 2 et 3, et pour 2 des 20 remorques (réactions équivoques) recevant le Traitement 4. Des virus viables on été détectés à l'aide du bio-essai utilisant des porcs ainsi qu'à l'aide des animaux sentinelles à partir des remorques du Traitement, et à l'aide du bio-essai utilisant des porcs seulement pour les écouvillons provenant des remorques du Traitement 4. Implications: Le lavage avec jet à haute pression des remorques de transport, suivi d'une exposition pendant 90 à 120 minutes à un désinfectant de type monopersulfate de potassium modifié ou un chlorure d'ammonium quaternaire appliqué avec un appareil moussant est susceptible d'éliminer les particules virales infectieuses résiduelles du PRRSV. |
Keywords: swine, disinfectant,
porcine reproductive and respiratory virus, transport, vehicle
Search the AASV web site
for pages with similar keywords.
Received: April
25, 2005
Accepted: July
7, 2005
Porcine reproductive and respiratory syndrome virus (PRRSV) is a single- stranded enveloped RNA virus classified in the order Nidovirales, family Arteriviridae, and genus Arterivirus.1 The disease caused by PRRSV, known as porcine reproductive and respiratory syndrome (PRRS), has proven to be very difficult to control consistently over time and across farms. A key component to successful control of PRRS is preventing spread of the virus within and between farms. Transmission of PRRSV can occur through a number of reported routes, including infected pigs, semen, contaminated fomites, insects, avian species, and aerosols.2-8 Another potential route of transmission between farms may be the livestock transport vehicle.9 In today's modern pig industry, the application of multi-site production technology has resulted in more movement of pigs and greater distances between sites and to slaughter. Therefore, pig transport has become a highly important risk factor for transmission of PRRSV. In support of this hypothesis, previously published reports10-12 have demonstrated how motorized vehicles can mechanically transport PRRSV over distances of 50 km, and specific assessments of the role of the transport vehicle in transmission of PRRSV have been conducted. In a recent study,12 1:150 scale models of weaned-pig trailers were used to enhance study power. The materials and designs used in these models were similar to those used in commercial transport vehicles, and the models provided for an animal density equal to that in a weaned-pig trailer capable of transporting 300 pigs. Under the conditions of that study,12 it was demonstrated that PRRSV-naive swine could become infected with PRRSV through contact with the contaminated interiors of the transport models. It was also determined that the concentration of PRRSV required to infect naive sentinel pigs was 1 x 103 median tissue culture infectious doses (TCID50) and that allowing the trailer to dry for 8 hours after washing effectively prevented infection in 10 of 10 replicates.12 However, discussion of these results with veterinarians working in large commercial systems indicated that sanitation programs requiring time periods > 2 hours limit the cost-effective use of trailers. Furthermore, accessibility to hot water (80°C) for washing was limited, and use of a low-pressure foaming system was a common method of applying disinfectant. Foam provided an effective vehicle to carry the disinfectant to the target surface and a means to hold it there in the short term. This technique has the added advantage of allowing the operator to see where the disinfectant has been applied.
Despite a growing interest in the use of foaming to apply disinfectants, there is little scientific evidence demonstrating its efficacy against PRRSV. While previous work had evaluated the ability of commercially available disinfectants to sanitize PRRSV-positive transport vehicles, the disinfectants had been applied by fogging, not foaming.13,14 Therefore, this study was conducted to test the principle of foaming in a sanitation protocol designed for PRRSV-positive commercial transport vehicles. To improve the authenticity of the study, the protocol incorporated several other methods frequently used in commercial swine systems, including cold water for washing and rapid turn-around time of trailers (< 2 hours). Turn-around time was defined as the time required to sanitize a contaminated trailer after animals were unloaded.
Materials and methods
Trailer models
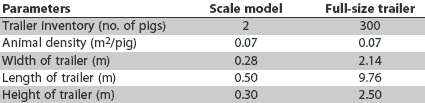
Figure 1: Four 1:150 models of full-size weaned-pig trailers were designed to allow for an equivalent animal density within the model trailer (2.5-kg pigs at 0.07 m2 per pig) compared to a full-sized trailer loaded to capacity (300 five-kg pigs). Dimensions are provided in Table 1.
|
Table 1: Dimensions and specifications of 1:150 scale-model single-deck aluminium trailers compared to full-size trailers commonly used to transport weaned pigs
|
To allow for multiple replications, the University of Minnesota Department of Biosystems and Agricultural Engineering constructed four 1:150 scale models (Figure 1) of full-size weaned-pig trailers.12-14 This scale allowed for an animal density within the model trailer (2.5-kg pigs at 0.07 m2 per pig) equivalent to that in a full-sized weaned-pig trailer loaded to capacity (300 five-kg pigs). As in full-size trailers, the frame, roof, and exterior sidewalls of the model trailers were flat aluminium, the flooring was polished aluminium tread-plate, and the interior walls were covered with textured styrene and insulated with foil-coated Styrofoam. Each exterior sidewall contained openings for proper ventilation, and a locking door was available on the end of each model. The dimensions of the full-size trailers and the models are provided in Table 1.
Experimental design
The study was conducted in two phases at the University of Minnesota Swine Disease Eradication Center (SDEC) research farm. In Phase One (detection of PRRSV RNA), each trailer model was deliberately contaminated with PRRSV, then assigned a specific disinfectant treatment. In Phase Two, infectivity of trailers was determined by swine bioassay and by housing naive sentinels in treated trailers.
Animal care and housing
All pigs were obtained from a source documented as PRRSV-naive on the basis of 10 years of clinical, diagnostic, and production data. All pigs were blood tested by polymerase chain reaction (PCR) and ELISA to confirm PRRSV-naive status upon arrival at the study site at 3 weeks of age.
Animals were cared for under the guidelines of the University of Minnesota Animal Care and Use Committee, which approved the study protocol. Animals were housed in commercial nursery pens with wire-mesh flooring and commercial feeders and waterers. Heat lamps were provided as needed, and fresh air was delivered by a negative-pressure power ventilation system. Animals were observed twice daily to insure that a comfortable living environment was in place.
Contamination of trailer models with PRRSV
The mechanically ventilated nursery room (25 m3) used for the study was heated to 20°C. Trailer models were placed in adjacent pens in the room (two trailers per pen). Trailer floors were covered with wood shavings, emulating a common practice in the North American seed-stock industry. As in the other investigations of PRRSV transmission by transport,10-14 the strain of PRRSV employed was a field isolate designated as MN 30-100.15 For each replicate, PRRSV was prepared at a concentration of 5 x 105 TCID50 in 5-mL aliquots of minimum essential medium (MEM; Difco Laboratories, Detroit, Michigan). Walls, ceilings, and floors of each trailer were inoculated using a hand-operated, multi-use power mister (Chapin Manufacturing, Batavia, New York). This high concentration of PRRSV was selected to exceed the previously determined concentration necessary to infect naive sentinel pigs housed in the model trailers, in order to thoroughly test disinfectant efficacy.12 After contamination, each trailer was assigned a specific treatment and placed in a pen in the nursery area, with maximum separation of the four pens housing the trailers during the treatment and intervention procedures.
Trailer washing and disinfectant treatment protocols
Treatments included washing with cold water alone (Treatment 1) and washing and then disinfecting with one of three disinfectants: modified potassium monopersulfate (Treatment 2); quaternary ammonium chloride (Treatment 3); and a phenolic disinfectant (Treatment 4). Swabs were collected from the interior of each trailer before and after treatment. A total of 20 replicates were conducted for each treatment, allowing for detection of a 48% reduction in the proportion of infected trailers at a target alpha level of 0.05 and an 80% study power.
In an effort to replicate protocols used in the field for transport time and sanitation of transport vehicles, data from an international breeding stock company (Genetiporc, Alexandria, Minnesota) were used throughout the study. This company sells breeding boars and gilts throughout North America and Latin America and operates approximately 15 transport vehicles, delivers approximately 1800 to 2000 truckloads of animals per year, and conducts approximately 30 to 35 sanitation procedures per week.
The interiors of all trailers were manually scraped with a hand-held plastic scraper to remove soiled bedding. To insure that mechanical transmission of PRRSV did not occur between treated and control trailers, the blade of the scraper was immersed in 70% ethanol, rinsed with sterile water, and swabbed between trailers. Trailers were then washed for 72 seconds using a commercial power washer (model TB5030A; American Made Cleaners, Beresford, South Dakota) that provided cold water (20°C) delivered at a pressure of 3000 psi. This wash time was extrapolated from the approximately 2-hour wash time for a full-sized weaned-pig trailer (R. Witt, Genetiporc, personal communication, April 2002).
Application of disinfectants
Bedding was removed and contaminated trailers were washed as described. No disinfectant was applied to trailers assigned Treatment 1. Disinfectants were applied to trailers assigned the other three treatments using a hydrofoamer (Hydro Systems Company, Cincinnati, Ohio) attached to a garden hose. Foam was applied to the interior of each trailer for 72 seconds, using a 1% solution of modified potassium monopersulfate (Treatment 2), and 30 mL of disinfectant per 3840 mL of water for the quaternary ammonium chloride product (Treatment 3) and the phenolic product (Treatment 4). For the control protocol, trailers were sham-disinfected using saline in the hydrofoamer.
Phase One: Detection of PRRSV RNA
Collection and testing of swabs. Before treatment (immediately after washing) and 30, 60, 90, and 120 minutes post treatment, swabs were collected from the interior of each trailer (floor, four walls, and ceiling, for a total of 0.14 cm2). For each trailer, a sterile swab (Dacron fiber-tipped plastic applicator swabs; Fisher Scientific Company, Hanover Park, Illinois) was moistened with MEM. Then, starting from the left side of each surface and progressing across the surface in a rightward direction, each swab was drawn over the walls, floor, and ceiling using a zigzag pattern. Swabs were then placed in sterile plastic tubes (Falcon, Franklin Lakes, New Jersey) containing 2 mL of MEM, and frozen at -20°C. When all required samples had been collected, swabs were tested for PRRSV RNA in duplicate using the TaqMan PCR assay (Perkin-Elmer Applied Biosystems, Foster City, California).16 The reaction was defined as "suspect" when one of the two PCR results was positive.
Phase Two: Infectivity of trailers
Swine bioassay. For this phase, a total of 16 pigs were employed. To prepare the bioassay samples,17 swabs in MEM were selected at the last sampling time when samples were PCR-positive, supernatant was removed and pooled by treatment, and 20 mL of MEM was added to each 20-sample pool. Each of the four treatment pools was divided into 5-mL aliquots that were injected IM (four pigs per treatment, 5 mL per pig). After injection, each pig was placed in an individual pen in a room assigned to the treatment group. Nose-to-nose contact was prevented. Biosecurity measures to prevent transmission of PRRSV between rooms4,18 included changing boots, gloves, and overalls between rooms, and 10-second immersion of boots in modified potassium mono-persulfate boot baths upon entering each room. Blood samples were collected from each pig before injection (Day 0) and on Days 7 and 14, and sera were tested for PRRSV RNA by PCR and for PRRSV antibodies by the Idexx 2X-R ELISA (Idexx Laboratories, Westbrook, Maine).
Sentinel exposure. Ninety minutes after completion of each trailer treatment replicate, two PRRSV-naive sentinel pigs were placed in each treated trailer for a 2-hour "transport" period (eight pigs total). This length of time was based on the mean time of 2 hours, reported by Genetiporc, required for a shipment of pigs to leave Site 1 (breeding, gestation, and farrowing farm) and arrive at Site 2 (nursery). After the 2-hour "transport" period, each sentinel pig was removed from the trailer and housed as described for bioassay pigs. Blood samples were collected from all sentinels before the 2-hour transport period began (Day 0) and on Days 7 and 14 and tested as described for the swine bioassay.
Controls
As validation that the methods used in treating the trailers did not accidentally contaminate the models, 20 replicates of a protocol control were conducted. For the purpose of this control, trailers were sham-inoculated with saline and swabbed as described. Finally, after each replicate of each treatment was completed, trailers were rewashed, hand-dried with disposable paper towels, and swabs were again collected for PCR testing to determine whether trailers were free of residual PRRSV RNA, in order to verify that each replicate was an independent event.
Statistical analysis
All treatments were tested using a one-tailed chi-square model, examining whether there was a significant difference in the number of positive samples over time within treatments.
Results
High-pressure washing did not completely remove organic debris from the trailers. Small amounts of residual bedding were visible in all trailers after the 72-second wash.
Phase One: PCR testing
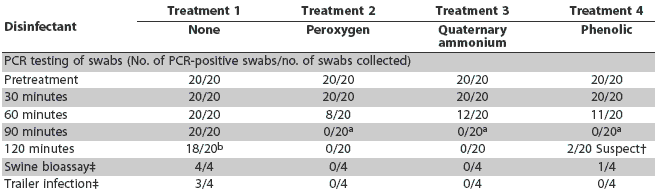
Results of PCR testing swabs collected from trailer interiors are summarized in Table 2. PRRS virus RNA was detected in all 20 replicates at 30, 60, and 90 minutes post treatment and in 18 of 20 replicates at 120 minutes in Treatment 1 (washing only). PRRS virus RNA was detected immediately post washing and at 30 minutes post treatment, but not at 90 and 120 minutes post treatment, in Treatment 2 (modified potassium monopersulfate) and Treatment 3 (quaternary ammonium chloride). Similar results were observed in Treatment 4 (phenolic) at 30, 60, and 90 minutes post treatment, but two suspect PCR reactions were detected 120 minutes post treatment. Protocol control samples were PCR-negative.
Phase Two: Detection of viable PRRSV
The results of the swine bioassay testing and the trailer infectivity assessments are also summarized in Table 2. All swab supernatants from trailers treated with modified potassium monopersulfate and quaternary ammonium chloride were negative on swine bioassay. In contrast, infectious PRRSV was detected in one of four samples collected from the sentinel pigs in trailers treated with the phenolic disinfectant and from four of four samples from pigs exposed to trailers treated with no disinfectant. Adverse side effects (irritation of the skin, abscess formation, swelling) were not detected at the site of injection of the bioassay sample, nor did pigs experience fever or loss of appetite.
Sentinels were infected in three of the four replicates for pigs housed in trailers not treated with disinfectant (Treatment 1), but in no replicates for Treatments 2, 3, and 4 (Table 2).
Table 2: Results of polymerase chain reaction (PCR), swine bioassay, and sentinel exposure testing after trailer models contaminated with porcine reproductive and respiratory syndrome virus (PRRSV) were pressure washed and either treated with a disinfectant or not treated*
* In all four treatments, 1:150-scale trailers were washed with water at 20°C, and disinfectants were applied with a hydrofoamer (1% peroxygen and 30 mL disinfectant/3840 mL of water for the quaternary ammonium chloride and phenolic products). One swab per treatment was collected from the interior of each trailer for PCR testing (20 replicates per treatment). For swine bioassay, four PRRSV-naive sentinel pigs per treatment were injected with supernatant from PCR-positive swabs collected at the latest post-treatment time when swabs were positive. For trailer infectivity, two sentinel were confined in each trailer for a 2-hour period beginning 90 minutes post treatment (four replicates per treatment, eight pigs total). Swine bioassay pigs and sentinel pigs were tested by serum PCR for PRRSV and by ELISA for PRRSV antibody on the day of injection or exposure, respectively, and 7 and 14 days later to determine a change in PRRS infection status. Results were defined as suspect when one of two duplicate PCR tests was positive. No. of replicates demonstrating PRRSV infection of naive sentinel pigs. a Difference significant (P < .01) when compared with result at previous sampling time (60 minutes). b Difference not significant (P = .24) when compared with result at previous sampling time (60 minutes). |
Washing alone (Treatment 1) had no statistically significant effect over time, with only a 10% decrease in number of positive samples at 120 minutes post disinfection. For Treatments 2 through 4, there were significantly fewer positive samples at contact times > 60 minutes compared to contact times <= 60 minutes. At 90 minutes, all samples were negative, and this was significantly less than at 60 minutes for all three disinfectants.
Discussion
The justification for this study was that scientific data on the efficacy of foaming for decontamination of PRRSV-positive transport vehicles were not available, and that this method of application of disinfectant was rapidly increasing in North American production systems. Under the conditions of the study, washing with cold water alone had little impact on eliminating PRRSV from trailer interiors, supporting previously published work.12-14 PRRS virus RNA was detected in 80 of 80 swabs (100%) collected immediately after the washing procedure across all replicates. Also, despite the use of high pressure, washing did not result in complete removal of organic debris from the trailers, a frequent observation under field conditions.
Results also indicated a difference in the performance of disinfectants tested in this study compared to previously published work.14 Samples from trailer models treated with modified potassium monopersulfate or quaternary ammonium chloride were negative for PRRSV RNA by PCR in all replicates at 90 and 120 minutes post treatment. No evidence of infectious PRRSV was detected in trailers treated with these products, as demonstrated by negative swine bioassay results and lack of seroconversion of sentinels post exposure to treated trailers. These results differ from previously reported results.14 One explanation may have been the use of a different concentration of the quaternary ammonium chloride product in this study (30 mL disinfectant per 3840 mL water) compared to the concentration of 15 mL disinfectant per 3840 mL water in the previous study.14 In addition, different means of applying the disinfectant were used (foaming and fogging).
In trailers treated with the phenolic product, PRRSV RNA was not detected at 90 minutes post treatment, but there were two suspect results 120 minutes post treatment in this treatment group. At the Minnesota Veterinary Diagnostic Laboratory, duplicate testing of each sample by PCR is standard protocol, and suspect results are reported if one of the two tests is positive. Again, these results differ from those previously reported for this product.14 Possible explanations could again include the difference in method of application (foaming and fogging), previously described differences in the concentration of the product, or the difference in room temperature under which the studies were conducted (20°C and 4°C). In contrast to results for the modified potassium monopersulfate and quaternary ammonium chloride treatments, one of four pooled swab supernatants from trailers treated with the phenolic product was bioassay-positive, indicating the presence of infectious PRRSV; however, sentinels housed in treated trailers were not infected. This finding confirms the presence of infectious PRRSV in the trailer interior and suggests differences in efficacy across the three products tested. However, sentinels housed in trailers may have failed to become infected because of an insufficient quantity of PRRSV, an inadequate number of replications conducted to detect the frequency of this event, or the inability of the pigs to access the virus due to the confines of the model.
This study contained several acknowledged limitations. Testing swabs by PCR alone was a primary limitation, as it was not possible to determine whether viable PRRSV was present in the trailer interior post treatment. This was countered by use of confirmatory tests, ie, live-pig exposure to treated trailers and swine bioassay to attempt to determine conclusively whether a positive PCR result was indicative of live or dead virus. Multiple factors may have contributed to negative PCR results, including the diagnostic sensitivity of the test, degradation of viral RNA in the sample through prolonged contact with the disinfectant, interference of the disinfectant with the PCR assay, or a truly efficacious disinfectant not only rendering the virus inactive but also degrading its nucleic acid. In the authors' opinion, test sensitivity did not appear to be an issue, as the TaqMan PCR assay is regularly able to detect PRRSV RNA in numerous samples, with a reported level of detection of 0.01 TCID50 per PCR reaction.16 The possibility of degradation of PRRSV RNA in swab samples secondary to prolonged contact with disinfectant during storage did not appear to be a problem, as shown by the large number of PCR-positive samples detected at 30 and 60 minutes post treatment across the various treatments. It was not possible in this study to add an agent to neutralise the disinfectant because of the potential virucidal effects of exogenous chemicals. Therefore, swabs were stored at -20°C immediately post collection to retard disinfectant activity,19-21 and underwent RNA extraction within 24 hours post collection, the standard practice in our previous studies on this subject.13,14 Potential interference of the individual disinfectants with the PCR assay did not appear to be an issue. PRRS virus RNA was detected in all replicates 30 minutes after application of treatments, indicating that it was possible for the TaqMan PCR assay to function properly in the presence of residual disinfectant.
Another acknowledged limitation of the study was inability to counteract the impact of drying that naturally occurred during the sampling period of 120 minutes. Other acknowledged limitations include inability to use full-size trailers and large loads of pigs. The models were scale models of weaned-pig trailers, and their construction design does not mimic a trailer that transports market animals, variables that certainly could impact the level of contamination in the trailer interior and the ease of cleaning. It is not known if the high concentration of PRRSV used to contaminate the trailers was representative of actual transport conditions. The entire interior of the models was contaminated, and this may not be representative of field conditions. It has been previously determined12 that sentinel pigs can be infected with PRRSV when these model trailers are contaminated with PRRSV concentrations of 1 x 103 TCID50. Therefore, in order to aggressively test the efficacy of the decontamination protocol, a high concentration of virus was selected as before. It must also be noted that the size of the swab was not proportional to the size of the model trailer and this may have impacted the recovery of PRRSV RNA. It was also not possible to conduct the study using market-age animals or adult breeding swine. Furthermore, although a relatively large number of replicates were conducted in Phase One, it was not sufficient to predict the frequency of the events recorded in the study. In addition, live animals were used in only four replicates; due to this small sample size, no estimation can be made regarding the frequency of the reported events. It was not possible to quantify the amount of PRRSV RNA present in PCR-positive samples. Finally, the results of this study cannot be directly extrapolated to other swine pathogens, such as transmissible gastroenteritis virus or Mycoplasma hyopneumoniae.
Despite its limitations, the study had considerable strength. It introduced use of a hydrofoamer for sanitation of PRRSV-contaminated surfaces and provided preliminary information on its efficacy against PRRSV. The equipment was easy to use, and its ability to provide visual confirmation of contact (ie, white foam) between the disinfectant and the surface will ensure better and more accurate application of disinfectant in repeated commercial usage. It showed that chemical sanitation using the modified potassium monopersulfate and the quaternary ammonium chloride products produced good inactivation of PRRSV within the target time when used with cold-water washing and application of disinfectant via foaming, further enhancing understanding of PRRSV sanitation protocols for commercial transport vehicles. While it was true that the trailer size and pig numbers were small, the models provided for an animal density equivalent to that of a full-size trailer. Furthermore, it would have been impossible to obtain full-size trailer loads (200 to 300 pigs) for even a single replicate, much less to repeat the study at any frequency. The use of industry standards for transport times and wash-water temperature and pressure, as well as commonly used disinfecting products and practices (ie, use of wood shavings) replicated real-world situations. One additional factor not included in the study design was use of detergents to facilitate removal of organic debris, and inclusion of such products might have enhanced the results and may decrease the time required for cleaning.
Implications
- These results will enable swine producers and practitioners to further understand and appreciate the merit of sanitizing livestock transport vehicles.
- Disinfectants may differ in their efficacy against PRRSV.
- Critical factors in sanitation program for PRRSV-contaminated transport vehicles include selecting an efficacious disinfectant, using it at the proper dilution rate and means of application, and allowing for sufficient contact time.
Acknowledgements
The authors would like to thank Dr Anna-Maria Castiglia-Zanello and Mr Paul Russell of Dupont Animal Health for technical assistance and financial support for this study.
References
1. Cavanagh N. Nidovirales. A new order comprising Coronoviridae and Arteriviridae. Arch Virol. 1997;142:629-633.
2. Dee SA, Yoon HS, Pijoan C. Controlling the spread of PRRS virus in the breeding herd through management of the gilt pool. Swine Health Prod. 1994;3:64-69.
3. Christopher-Hennings J, Nelson EA, Hines RJ, Nelson JK, Swenson SL, Zimmerman JJ, Chase CCL, Yaeger MJ, Benfield DA. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J Vet Diagn Invest. 1995;7:456-464.
4. Otake S, Dee SA, Rossow KD, Joo HS, Deen J, Molitor TW. Transmission of porcine reproductive and respiratory syndrome virus by fomites. J Swine Health Prod. 2002;10:59-65.
5. Otake S, Dee SA, Rossow KD, Moon RD, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by mosquitoes. Can J Vet Res. 2002;66:191-195.
6. Otake S, Dee SA, Rossow KD, Moon RD, Trincado C, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica). Vet Rec. 2003;152:73-76.
7. Zimmerman JJ, Yoon KJ, Pirtle FC, Wills RW, Sanderson TJ, McGinley MJ. Studies of porcine reproductive and respiratory syndrome virus in avian species. Vet Microbiol. 1997;55:329-336.
8. Torremorell M, Pijoan C, Janni K, Walker R, Joo HS. Airborne transmission of Actinobacillus pleuropneumoniae and porcine reproductive and respiratory syndrome virus in nursery pigs. Am J Vet Res. 1997;58:828-832.
9. Poumian E. Disinfection of trucks and trailers. Rev Sci Tech Off Int Epiz. 1995;14:171-176.
10. Dee SA, Deen J, Rossow KD, Mahlum C, Otake S, Joo HS, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during cold weather. Can J Vet Res. 2002;66:232-239.
11. Dee SA, Deen J, Rossow KD, Mahlum C, Eliason R, Otake S, Joo HS, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during warm weather. Can J Vet Res. 2003;67:12-16.
12. Dee SA, Deen J, Otake S, Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can J Vet Res. 2004;68:128-133.
13. Dee SA, Deen J, Burns D, Douthit G, Pijoan C. An assessment of sanitation protocols for commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:208-214.
14. Dee SA, Deen J, Pijoan C. Evaluation of disinfectants for the sanitation of porcine reproductive and respiratory syndrome virus-contaminated transport vehicles at cold temperatures. Can J Vet Res. 2005;69:64-70.
15. Bierk MD, Dee SA, Kossow KD, Collins JE, Guedes MI, Pijoan C, Molitor TW. Diagnostic investigation of chronic PRRS virus infection in a breeding herd of pigs. Vet Rec. 2001;148:687-690.
*16. Molitor TW, Tune KA, Shin J, Collins J, Kapur V. Applications of TaqMan PCR in the detection of porcine reproductive and respiratory syndrome virus. Proc AD Leman Swine Conf. Brooklyn Center, Minnesota. 1997;173-175.
17. Swenson SI, Hill HT, Zimmerman JJ, Evans LE, Landgraf JG, Wills RW, McGinely M, Brevik AK, Ciszewski D, Frey ML. Excretion of porcine reproductive and respiratory syndrome virus after experimentally induced infection in boars. JAVMA. 1994;204:1943-1948.
18. Dee SA, Deen J, Pijoan C. An evaluation of 4 intervention strategies to prevent mechanical transmission of porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:19-26.
19. Russell AD, Hugo WB. Chemical disinfectants. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. 1st ed. Oxford, England: Blackwell Scientific Publications Ltd; 1987:12-42.
20. Quinn PJ. Evaluation of veterinary disinfectant and disinfection processes In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. 1st ed. Oxford, England: Blackwell Scientific Publications Ltd; 1987:66-116.
21. Morgan-Jones S. Practical aspects of disinfection and infection control. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. 1st ed. Oxford, England: Blackwell Scientific Publications Ltd; 1987:144-167.
* Non-refereed reference.