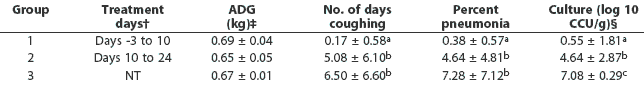Original research |
Peer reviewed |
Efficacy of a chlortetracycline feed additive in reducing pneumonia and clinical signs induced by experimental Mycoplasma hyopneumoniae challenge
Eficiencia de la clorotetraciclina como aditivo en el alimento para reducir la neumonía y los signos clínicos inducidos por el reto experimental con Mycoplasma hyopneumoniae
Efficacité d'un supplément alimentaire à base de chlortétracycline à réduire les signes cliniques et les lésions de pneumonie suite à une infection expérimentale par Mycoplasma hyopneumoniae
Eileen L. Thacker, DVM, PhD, Diplomate ACVM; Brad J. Thacker, DVM, PhD, MBA, Diplomate ABVP; Teddi Wolff, DVM, MS
ELT: Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa. BJT: Department of Veterinary Diagnostics and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa. TW: Alpharma Inc, Fort Lee, New Jersey. Corresponding author: Dr Eileen L. Thacker, 2118 Veterinary Medical Building, Department of Veterinary Microbiology and Preventive Medicine, College of Veterinary Medicine, Iowa State University, Ames, IA 50011; Tel: 515-294-5097; Fax: 515-294-8500; E-mail: ethacker@iastate.edu.
Cite as: Thacker EL, Thacker BJ, Wolff T. Efficacy of a chlortetracycline feed additive in reducing pneumonia and clinical signs induced by experimental Mycoplasma hyopneumoniae challenge. J Swine Health Prod. 2006;14(3):140-144.
Also available as a PDF.
SummaryObjectives: To determine the efficacy of a chlortetracycline (CTC) feed additive on pneumonia and clinical signs induced by Mycoplasma hyopneumoniae in an experimental challenge model. Methods: Three groups of pigs (12 pigs per group) were challenged with M hyopneu-moniae (Day 0). Two groups received feed containing CTC at 550 g per tonne (500 g per ton; 22 mg per kg of bodyweight) for 14 days, starting either on Day -3 (prior to challenge) or at onset of clinical signs (Day 10). Pigs were evaluated daily for clinical disease (coughing), and all were necropsied on Day 29. Percentage of lung affected by pneumonia, number of organisms isolated from lung tissue, and serum antibodies (ELISA) were measured. Results: Pigs fed CTC starting before inoculation had significantly fewer coughing days and less pneumonia than either of the other groups. Pigs in both CTC-medicated groups had significantly fewer M hyopneu-moniae organisms at necropsy than non-medicated pigs. At necropsy, 50% of control pigs were seropositive for M hyopneumoniae antibodies, while none of the CTC-treated pigs had seroconverted. Implications: Under the conditions of this study, in pigs challenged with M hyopneu-moniae, less severe clinical signs and pneumonia occur and fewer organisms may be isolated from lung tissue when treatment with CTC begins before challenge rather than after the onset of clinical signs. In addition, fewer organisms may be isolated from lung tissue of pigs treated with CTC beginning with the onset of clinical signs, compared to untreated pigs. In-feed CTC may be effective against mycoplasmal pneumonia. | ResumenObjetivos: Determinar la eficacia de la clorotetraciclina (CTC por sus siglas en inglés) contra la neumonía y signos clínicos inducidos por el Mycoplasma hyopneumoniae en un modelo de reto experimental. Métodos: Tres grupos de cerdos (12 cerdos por grupo) se retaron con M hyopneumoniae (Día 0). Dos grupos recibieron alimento que contenía CTC a 550 gr por tonelada métrica (500 gr por tonelada corta; 22 mg por kilo de peso corporal) por 14 días, iniciando el Día -3 (antes del reto) o al inicio de los signos clínicos (Día 10). Los cerdos fueron evaluados diariamente en busca de signos clínicos (tos), y todos se sacrificaron el Día 29. Se midieron el porcentaje de pulmón afectado por neumonía, el número de organismos aislados del tejido pulmonar, y los anticuerpos en suero (ELISA). Resultados: Los cerdos alimentados con CTC antes de la inoculación, presentaron significativamente menos días con tos y menos neumonía que cualquiera de los otros grupos. Los cerdos en ambos grupos medicados con CTC presentaton significativamente menos organismos de M hyopneumoniae a la necropsia que los cerdos no medicados. A la necropsia, 50% de los cerdos control fueron seropositivos a anticuerpos contra M hyopneumoniae, mientras que ninguno de los cerdos tratados con CTC seroconvirtieron. Implicaciones: Bajo las condiciones de este estudio, en los cerdos retados con M hyopneumoniae, hubo menos neumonía y signos clínicos severos y menos organismos fueron aislados del tejido pulmonar cuando el tratamiento con CTC inició antes del reto, a diferencia que después del inicio de los signos clínicos. Además, se puedieron aislar menos organismos del tejido pulmonar de cerdos tratados con CTC cuando esta es suministrada al inicio de los signos clínicos, en comparación con los cerdos no tratados. La CTC en alimento puede ser efectiva contra la neumonía causada por mycoplasma. | ResuméObjectifs: Déterminer l'efficacité d'un additif alimentaire contenant de la chlortétracycline (CTC) à réduire les signes cliniques et les lésions de pneumonie causés dans un modèle expérimental d'infection par Mycoplasma hyopneumoniae. Méthodologie: Trois groupe de porcs (12 porcs par groupe) ont été inoculés avec M hyopneumoniae (Jour 0). Deux groupes ont reçu un aliment contenant de la CTC à un dosage de 550 g par tonne métrique (500 g par tonne; 22 mg par kg de poids corporel) pendant 14 jours, débutant soit au Jour -3 (avant l'inoculation) soit lors de l'apparition des signes cliniques (Jour 10). Une évaluation quotidienne des animaux a été faite pour la présence de maladie clinique (toux) et tous ont été soumis à une nécropsie au Jour 29. Le pourcentage de poumon présentant des lésions de pneumonie, le nombre de micro-organismes isolés du tissu pulmonaire, et les titres d'anticorps sériques détectés par ELISA ont été mesurés. Résultats: Les porcs recevant de la CTC avant l'inoculation ont eu significativement moins de jours avec de la toux et moins de lésion de pneumonie que chacun des deux autres groupes. Lors de la nécropsie, on retrouvait significativement moins de M hyopneumoniae chez les porcs dans les deux groupes ayant reçu de la CTC que chez les porcs non-médicamentés. Au moment de la nécropsie, 50% des porcs témoins avaient des anticorps contre M hyopneumoniae alors qu'aucun des animaux traités à la CTC n'avait d'anticorps contre M hyopneumoniae. Implication: Dans les conditions expérimentales de cette étude, des porcs inoculés avec M hyopneumoniae avaient moins de signes cliniques, moins de lésions de pneumonie et moins de micro-organismes ont été isolés des poumons lorsque le traite-ment à la CTC a débuté avant l'inoculation plutôt qu'après l'apparition des signes cliniques. De plus, moins de micro-organismes ont été isolés du tissu pulmonaire de porcs traités avec de la CTC au début de l'apparition des signes cliniques comparativement à des animaux non-traités. De la CTC ajoutée aux aliments peut être efficace contre la pneumonie à mycoplasme. |
Keywords: swine, Mycoplasma
hyopneumoniae, chlortetracycline
Search the AASV web site
for pages with similar keywords.
Received: April
5, 2005
Accepted: June
21, 2005
Mycoplasma hyopneumoniae, the cause of enzootic pneumonia, affects many swine herds in the United States.1 Mycoplasma hyopneumoniae is minimally pathogenic by itself; however, it is commonly isolated from pigs showing clinical signs associated with the porcine respiratory disease complex (PRDC), which is characterized by respiratory disease, fever, anorexia, and lethargy. Although multiple pathogens are isolated from pigs exhibiting PRDC, M hyopneumoniae appears to play an important role in the underlying pneumonia.2 The interaction between M hyopneumoniae and swine viruses, including porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2), is complex, and previous studies in our laboratory have found that infection with M hyopneumoniae increases the duration and severity of pneumonia induced by these viruses.3,4
Antibiotic therapy in conjunction with vaccination is considered an important intervention strategy for treatment and control of mycoplasmal pneumonia. A number of studies have assessed vaccine efficacy and the effectiveness of several antibiotics in production settings.5-9 These studies have used production parameters to assess the efficacy of the products; however, the ability to reduce the number of M hyopneumoniae organisms in vivo remains poorly defined. Currently, only one feed-grade medication (Lincomix; Pfizer Inc, Exton, Pennsylvania) is approved for mycoplasmal pneumonia, and the label claim is for reduction of severity of pneumonia, not the number of M hyo-pneumoniae organisms. In addition, several studies have found that antibiotics such as tiamulin appear to have a high activity against M hyopneumoniae in vitro; however, conflicting results have been observed in vivo.7,10-12
An earlier challenge study and an in vitro study suggested that chlortetracycline (CTC) was effective against M hyopneumoniae.11,13 Chlortetracycline (22 mg per kg of body weight in feed for up to 14 days) is approved for treatment of swine bacterial pneumonia caused by Pasteurella multocida. The objective of the current study was to assess the efficacy of CTC against M hyopneumoniae, as determined by the percentage of lung tissue affected by pneumonia, incidence of clinical disease as measured by coughing, development of serum antibodies to M hyopneumoniae, and number of mycoplasma organisms isolated from the respiratory tracts of experimentally infected pigs.
Materials and methods
Animals and housing
Thirty-six crossbred, castrated male pigs, approximately 10 to 12 days of age upon arrival, were procured from a breed-to-wean swine herd serologically negative for M hyopneumoniae and PRRSV. The study was approved and conducted in accordance with the guidelines of the Iowa State University Institutional Committee on Animal Care and Use. Each treatment group consisted of 12 pigs, which were housed in separate rooms within the Livestock Infectious Disease Isolation Facilities at Iowa State University. All rooms had similar lighting and environmental conditions. Pigs were placed in decked pens (1.24 m x 1.24 m) with three pigs per pen. Feeders and nipple waterers in each pen allowed ad libitum feed and water intake throughout the study.
Study design
The pigs were acclimated for approximately 3 weeks prior to study initiation, and were randomly assigned to three treatment groups with stratification by weight. On Day 0, all pigs were inoculated intratracheally with a tissue homogenate containing M hyopneumoniae strain 232 (a derivative of strain 11), 105 color-changing units (CCU) per mL, at a dilution of 1:100 in 10 mL of Friis medium as previously described.14
Group 1 received CTC-medicated feed beginning on Day -3, before the pigs were inoculated with M hyopneumoniae, and until 10 days post inoculation. Group 2 was provided medicated feed at onset of clinical signs (coughing) beginning Day 10 and until Day 24. Group 3 received nonmedicated feed throughout the duration of the study.
All pigs were observed daily for 15 minutes for coughing. Coughing scores were calculated as the number of days each pig was observed coughing. Blood samples for antibody assessment were obtained prior to the study and at necropsy. Pigs were individually weighed and average daily gain (ADG) was calulated weekly throughout the study.
Feed and administration of CTC
For the first 2 weeks post arrival, all pigs were fed a commercial pelleted diet containing 55 g per tonne (50 g per ton) carbadox (Mecadox; Phibro Inc, Fort Lee, New Jersey). Six days prior to addition of CTC, the diet was changed to a nonmedicated meal. The medicated meal was nutritionally formulated the same as the control diet and contained CTC (Aureomycin 50 Granular premix, 50 g CTC per lb of premix [110 g per kg]; Alpharma Inc, Fort Lee, New Jersey) at the rate of 550 g per tonne (500 g per ton), to provide 22 mg CTC per kg of body weight. Representative samples of medicated and nonmedicated diets were collected in plastic bags and stored at -20°C until assayed to confirm medication levels.
Serology
Serum samples were tested using a Tween-20 ELISA as previously described.15 Known positive and negative sera, as well as blank wells, were included as controls on each plate. Samples with values > 2 standard deviations (SD) above the mean optical density (OD) value of the negative control sera were considered positive (OD >= 0.200).
Necropsy
On Day 29, pigs were anesthetized with sodium pentobarbital administered intravenously and were exsanguinated. Tissue samples were collected aseptically from the lungs for culture. The bronchi were swabbed for bacterial culture and M hyopneumoniae isolation. Lesions consistent with mycoplasmal pneumonia were sketched onto standard lung diagrams, and the proportion of lung area with pneumonia lesions was determined as previously described.16 Lung tissue was frozen for fluorescent antibody (FA) assay examination for mycoplasmal antigen.17
Microbiology
Bronchial swabs were cultured for respiratory pathogens, including Bordetella bronchiseptica, Pasteurella multocida, Actinobacillus species, Haemophilus species, and Mycoplasma hyorhinis. Samples were inoculated onto blood agar and streaked with a Staphylococcus epidermidis nurse colony for support of Actinobacillus pleuropneumoniae and Haemophilus species. Isolates were identified according to standard methods.
Mycoplasma hyopneumoniae isolation and titration
To confirm the presence of M hyopneumoniae in the respiratory tract, bronchial secretions were inoculated onto Friis medium. Colonies having morphology consistent with M hyo-pneumoniae were identified by epiimuno-fluorescence with conjugates prepared from rabbit antisera to M hyopneumoniae.18 For determining the number of M hyopneumoniae organisms, approximately 1 g of lung tissue was processed and titrated as previously described.19 Briefly, lung tissue was collected aseptically and ground in Friis medium with no added antibiotics. Aliquots of the tissue homogenate were inoculated into Friis medium with methicillin and bacitracin and serially diluted in the same medium through seven tenfold dilutions. The number of organisms was reported as CCU per g of lung tissue.
Statistical analysis
Analysis of variance was performed using the general ANOVA procedure of Statistix (Analytical Software, Tallahassee, Florida) to ascertain differences in mean ADG between treatment groups. If the P value generated by ANOVA was P < .05, pair-wise comparisons among the three groups were performed by least significant difference at a significance level of P < .05. Kruskall-Wallis ANOVA was used for nonparametric analysis (mean number of coughing days, mean percent of lung affected by pneumonia, and mean number of organisms cultured from lung tissue).
Results
Growth and health
No differences in ADG were observed among the groups (Table 1). Pigs in Groups 2 and 3 began coughing at approximately Day 7. Only one pig in Group 1 was heard coughing during the trial (Table 1). The average percentage of pneumonia was significantly less in Group 1 than in the other two groups (Table 1). No statistical sdifference between Group 2 and Group 3 pigs was observed in the average percent pneumonia (Table 1).
Table 1: Means (+/- SD) for average daily gain (ADG), number of coughing days, percent of lung affected by pneumonia, and number of organisms isolated from lung tissue in 36 pigs challenged with Mycoplasma hyopneumoniae at 4 to 5 weeks of age and either treated with chlortetracycline (CTC) or not treated*
* Three groups of 12 pigs housed in separate rooms were inoculated intratracheally with M hyopneumoniae on Day 0. Pigs were observed for coughing for 15 minutes daily, and the number of days each pig was observed coughing was calculated. The proportion of lung area with pneumonia lesions was determined by image analysis at necropsy on Day 29. † CTC was administered in feed at 22 mg per kg body weight daily. ‡ Pigs were weighed weekly between Day -3 and Day 29. § Titration of M hyopneumoniae, log 10 color-changing units (CCU)/g lung tissue. abc Values with different superscripts within a column are significantly different (ANOVA; P < .05). NT = not treated |
Microbiology, fluorescent antibody, and serology results
No pathogenic bacteria associated with respiratory disease, other than M hyopneu-moniae, were isolated from the respiratory tracts of any pigs. The numbers of M hyo-pneumoniae organisms (CCU per g of lung tissue) were lower (P < .05) in Groups 1 and 2 (both treated with CTC) than in Group 3 (no CTC treatment) (Table 1). In addition, the number of organisms in lung tissue was lower in Group 1 (treated beginning Day -3) than in Group 2 (treated beginning on Day 10) (P < .05).
Mycoplasma hyopneumoniae antigens, as determined by FA, were detected in one of 12 pigs (8%) in Group 1, five of 12 pigs (42%) in Group 2, and all 12 pigs in Group 3.
Prior to challenge, all pigs were serologically negative, with an overall group average OD of 0.005 +/- 0.006. At the end of the trial (Day 29), six of the 12 Group 3 pigs (50%) were seropositive for M hyopneumoniae antibodies, while no pigs that had received CTC medication (Groups 1 and 2) were seropositive (0%).
Discussion
An effective M hyopneumoniae control option should ideally lessen pneumonic damage in the animal as well as reducing numbers of M hyopneumoniae organisms or eliminating them. Vaccination is a frequent and often effective intervention strategy for controlling the pneumonia induced by M hyopneumoniae; however, vaccines do little to reduce colonization.20-23 In addition, vaccine failure may occur with commercial mycoplasma vaccines due to concurrent infection with PRRSV, persisting maternally derived antibodies, or both.24,25
In many cases, strategic use of antibiotics is required to assist in controlling respiratory disease associated with M hyopneumoniae infection as well as other bacterial infections. While M hyopneumoniae is sensitive to antibiotics in vitro, less is known about antibiotic efficacy in vivo.6-12,26 Lincomix (Pfizer, New York, New York) is approved at 220 g per tonne of feed for reducing the severity of mycoplasmal pneumonia; however, no information is available on its effect on numbers of M hyopneumoniae organisms. A previous in vivo study demonstrated that CTC was effective in reducing the level of pneumonia induced by M hyopneumoniae.13 However, the effect on organism numbers was not determined in that study. European studies demonstrated that feeding either Lincomix or tilmicosin improved production parameters associated with mycoplasmal pneumonia.8,9 However, those studies did not directly assess either reduction of mycoplasmal pneumonia or effect of the antibiotics on numbers of M hyopneumoniae organisms in the respiratory tract. In addition, recent research has suggested that antimicrobial resistance may be a problem with some M hyopneumoniae field isolates.27
Production parameters have been used as measures of antibiotic efficacy under field conditions. However, in many cases, the improved production parameters associated with antibiotic therapy may be due to efficacy of the antimicrobial on other organisms present concurrently with M hyopneumoniae. While this type of information is important to practitioners and producers, identifying the effect of an antibiotic on M hyopneumoniae is also important, especially if eradication is a goal for antibiotic use. In the current study, while the numbers of M hyopneumoniae organisms were significantly lower in groups treated with CTC, treatment did not completely eliminate M hyopneumoniae from these pigs. Thus, it is possible that when the antibiotic is withdrawn, M hyopneumoniae organisms may increase in number and may cause clinical disease at a later date. In this study, although Group 1 pigs received no medication for 19 days before necropsy, numbers of M hyopneumoniae organisms were still significantly lower in Group 1 than in the other two groups, indicating that the number of M hyopneumoniae organisms, as measured by CCU per g of lung tissue, did not increase significantly immediately after withdrawal of the CTC. This was further confirmed by fewer organisms detected by FA testing. In addition to the lower number of organisms observed in the respiratory tracts of pigs in Group 1, the low incidence of coughing in this group would also minimize organism transmission between pigs. While it was observed that pigs treated with CTC failed to seroconvert, it is unknown whether they remained immunologically naive. As serum antibodies against M hyopneumoniae are not correlated with protection against disease,16-28 the importance of lack of seroconversion is unknown. It could be hypothesized that, while CTC may not eliminate M hyopneu-moniae from the pig, use of CTC in conjunction with vaccination, especially in nursery pigs, might provide sufficient time for a mature and active immune response to develop after vaccination, while decreasing the potential for clinical mycoplasmal pneumonia throughout the production period.
Future studies might include assessment of CTC efficacy in nursery-age pigs in which other factors, such as PRRSV infection or maternal antibodies against M hyopneumo-niae, may interfere with M hyopneumoniae vaccine efficacy.24,25 The findings of this study suggest that CTC is beneficial in controlling and reducing mycoplasmal pneumonia.
Implications
- Under the conditions of this study, in pigs challenged with M hyopneumoniae, amount of coughing, proportion of lung affected with pneumonia lesions, and numbers of M hyopneumoniae organisms in lung tissue are lower in animals treated with CTC beginning before challenge than in nonmedicated pigs.
- Under the conditions of this study, numbers of M hyopneumoniae organisms in lung tissue are lower in pigs treated with CTC after the onset of clinical disease (coughing) than in nonmedicated pigs.
- Although not labelled for this use, in-feed CTC may be beneficial in controlling and reducing mycoplasmal pneumonia.
Acknowledgements
This work was supported by a research grant from Alpharma Inc. The authors would like to thank Barbara Erickson, Nancy Upchurch, and the students in the Thacker laboratory for their technical assistance in this study.
References
1. National Center for Animal Health Surveillance. National Animal Health Monitoring Survey 2000. Available at: www.aphis.usda.gov/vs/ceah/cahm. Accessed January 12, 2006.
2. Halbur PG. Defining the causes of PRDC. Swine Consultant. 1996;fall:4-15.
3. Opriessnig T, Thacker EL, Yu S, Fenaux M, Meng X-J, Halbur P. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet Pathol. 2004;41:624-640.
4. Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker B. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 1999;37:620-627.
5. Hsu FS, Yeh TP, Lee CT. Tiamulin feed medication for the maintenance of weight gains in the presence of mycoplasmal pneumonia in swine. J Anim Sci. 1983;57:1474-1478.
6. Hannan PC, Goodwin RF. Treatment of experimental enzootic pneumonia of the pig by norfloxacin or its 6-chloro analogue. Res Vet Sci. 1990;49:203-210.
7. Hannan PC, Bhogal BS, Fish JP. Tylosin tartrate and tiamutilin effects on experimental piglet pneumonia induced with pneumonic pig lung homogenate containing mycoplasmas, bacteria and viruses. Res Vet Sci. 1982;33:76-88.
8. Mateusen B, Maes D, Hoflack G, Verdonck M, de Kruif A. A comparative study of the preventive use of tilmicosin phosphate (Pulmotil premix) and Mycoplasma hyopneumoniae vaccination in a pig herd with chronic respiratory disease. J Vet Med B, Infect Dis Vet Pub Health. 2001;48:733-741.
9. Mateusen B, Maes D, Van Goubergen M, Verdonck M, de Kruif A. Effectiveness of treatment with lincomycin hydrochloride and/or vaccination against Mycoplasma hyopneumoniae for controlling chronic respiratory disease in a herd of pigs. Vet Rec. 2002;151:135-140.
10. Hannan PC, Windsor HM, Ripley PH. In vitro susceptibilities of recent field isolates of Mycoplasma hyopneumoniae and Mycoplasma hyosynoviae to valnemulin (Econor), tiamulin and enrofloxacin and the in vitro development of resistance to certain antimicrobial agents in Mycoplasma hyopneumoniae. Res Vet Sci. 1997;63:157-160.
11. Wu CC, Shryock TR, Lin TL, Fleck M. Testing antimicrobial susceptibility against Mycoplasma hyopneumoniae in vitro. J Swine Health Prod. 1997;5:227-230.
12. Ross RF, Cox DF. Evaluation of tiamulin for treatment of mycoplasmal pneumonia in swine. JAVMA. 1988;193:441-446.
*13. Underdahl NR, New CW. Reducing the lesions of mycoplasmal pneumonia in gnotobiotic pigs with chlortetracycline. Proc 24th Ann Geo A Young Conf. 1983;29-36.
14. Friis NF. Some recommendations concerning primary isolations of Mycoplasma suipneumoniae and Mycoplasma flocculare. Nordic Vet Med. 1975;27:337-339.
15. Bereiter M, Young TF, Joo HS, Ross RF. Evaluation of the ELISA and comparison to the complement fixation test and radial immunodiffusion enzyme assay for detection of antibodies against Mycoplasma hyopneumoniae in swine serum. Vet Micro. 1990;25:177-192.
16. Thacker EL, Thacker BJ, Boettcher TB, Jayappa H. Comparison of antibody production, lymphocyte stimulation, and protection induced by four commercial Mycoplasma hyopneumoniae bacterins. J Swine Health Prod. 1998;6:107-112.
17. Amanfu W, Weng CN, Ross RF, Barnes HJ. Diagnosis of mycoplasmal pneumonia of swine: sequential study by direct immunofluorescence. Am J Vet Res. 1984;45:1349-1352.
18. Del Giudice RA, Robillard NF, Carski TR. Immunofluorescence identification of mycoplasma on agar by use of incident illumination. J Bact. 1967;93:1205-1209.
19. Thacker EL, Thacker BJ, Kuhn M, Hawkins PA, Waters WR. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Am J Vet Res. 2000;61:1384-1389.
20. Dohoo IR, Montgomery ME. A field trial to evaluate a Mycoplasma hyopneumoniae vaccine: effects on lung lesions and growth rates in swine. Can Vet J. 1996;37:299-302.
21. Jensen CS, Ersboll AK, Nielsen JP. A meta-analysis comparing the effect of vaccines against Mycoplasma hyopneumoniae on daily weight gain in pigs. Prev Vet Med. 2002;54:265-278.
22. Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Lein A, Virjens B, de Kruif A. The effect of vaccination against Mycoplasma hyopneumoniae in pig herds with a continuous production system. Zentralbl Veterinarmed B. 1998;45:495-505.
23. Maes D, Deluyker H, Verdonck M, Castryck F, Miry C, Lein A, Virjens B, Verbeke W, Viaene J, de Kruif A. Effect of vaccination against Mycoplasma hyopneumoniae in pig herds with an all-in/all-out production system. Vaccine. 1999;17:1024-1034.
*24. Thacker BJ, Thacker EL. Influence of maternally-derived antibodies on the efficacy of a Mycoplasma hyopneumoniae bacterin. Proc AASV. Nashville, Tennessee. 2001;513-515.
25. Thacker EL, Thacker BJ, Young TF, Halbur PG. Effect of vaccination on the potentiation of porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia by Mycoplasma hyopneumoniae. Vaccine. 2000;18:1244-1252.
26. Hannan PC, O'Hanlon PJ, Rogers NH. In vitro evaluation of various quinolone antibacterial agents against veterinary mycoplasmas and porcine respiratory bacterial pathogens. Res Vet Sci. 1989;46:202-211.
27. Stakenborg T, Vicca J, Butaye P, Maes D, Minion FC, Peeters J, de Kruif A, Haesbrouck F. Characterization of in vivo acquired resistance of Mycoplasma hyopneumoniae to macrolides and lincosamides. Microb Drug Resist. 2005;11:290-294.
28. Djordjevic SP, Eamens GJ, Romalis LF, Nichols PJ, Taylor V, Chin J. Serum and mucosal antibody responses and protection in pigs vaccinated against Mycoplasma hyopneumoniae with vaccines containing a denatured membrane antigen pool and adjuvant. Aust Vet J. 1997;75:504-511.
* Non-refereed references.