Original research |
Peer reviewed |
Evaluation of an industry-based sanitation protocol for full-size transport vehicles contaminated with porcine reproductive and respiratory syndrome virus
Evaluación de un protocolo utilizado en la industria para el lavado de vehículos de transporte contaminados con el virus del síndrome reproductivo y respiratorio porcino
Évaluation d’un protocole de désinfection des véhicules de transport commerciaux contaminés par le virus du syndrome reproducteur et respiratoire du porc
S. A. Dee, DVM, MS, PhD, Diplomate ACVM; John Deen, DVM, MS, PhD, Diplomate ABVP; Carlos Pijoan, DVM, PhD
SC: Swine Disease Eradication Center, University of Minnesota College of Veterinary Medicine, St Paul, Minnesota. JD, CP: Department of Veterinary Population Medicine, College of Veterinary Medicine, University of Minnesota, St Paul, Minnesota. Corresponding author: Dr Scott Dee, Swine Disease Eradication Center, University of Minnesota, College of Veterinary Medicine, Room 385C, 1988 Fitch Avenue, St Paul, MN 55108; Tel: 612-625-4786; Fax: 612-625-1210; E-mail: deexx004@umn.edu.
Cite as: Dee SA, Deen J, Pijoan C. Evaluation of an industry-based sanitation protocol for full-size transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2006;14(6):307–311.
Also available as a PDF.
SummaryObjective: To test a protocol for sanitation of full-size commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus (PRRSV), utilizing conditions found on commercial swine production units. Conditions included use of cold water for washing (21°C), application of a commercial disinfectant via a low-pressure foamer, and rapid completion of ≤ 2 hours. Materials and methods: Fifteen sites in a trailer were experimentally contaminated with IngelVac PRRS MLV vaccine (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri; total of 5 × 105 median tissue culture infectious doses per site). Ten replicates were conducted. The presence or absence of PRRSV RNA was evaluated by polymerase chain reaction (PCR) testing of swabs taken from the trailer’s interior before treatment and 120 minutes post treatment. Swabs that were PCR-positive were then evaluated for viable PRRSV by swine bioassay. Treatment consisted of washing with cold water then disinfecting with a 1% solution of modified potassium monopersulfate applied via low-pressure foaming. The trailer was not dried. Results: In 10 of 150 samples collected across the 10 replicates, PRRSV RNA was detected 120 minutes post treatment. Differences in the percentages of PCR-positive swabs collected at 0 and 120 minutes post treatment in treatment and control replicates were significant (P < .001; Fisher’s exact test). Viable virus was not detected by swine bioassay. Implication: High-pressure washing of transport trailers, followed by 120 minutes exposure to 1% modified potassium monopersulfate applied with a hydrofoamer, will most likely eliminate residual infectious PRRSV. | ResumenObjetivo: Probar un protocolo para el lavado de vehículos de transporte comerciales contaminados con el virus del síndrome reproductivo y respiratorio porcino (PRRSV por sus siglas en inglés), utilizando condiciones actuales encontradas en las unidades de producción porcina comercial. Las condiciones incluyeron el uso de agua fría para lavar (21°C), la aplicación de un desinfectante comercial a través de un espumador de baja presión, y un tiempo de proceso rápido de ≤ 2 horas. Materiales y métodos: Se contaminaron experimentalmente quince sitios en un trailer con la vacuna IngelVac PRRS MLV (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri; un total de 5 × 105 dosis infecciosas promedio en cultivo celular por sitio). Se realizaron diez réplicas. La presencia o ausencia del RNA del PRRSV se evaluó mediante la reacción en cadena de polimerasa (PCR por sus siglas en inglés) de los hisopos tomados del interior del trailer antes del tratamiento y 120 minutos después del tratamiento. Los hisopos que fueron PCR positivos se evaluaron en busca de PRRSV viable mediante el bioensayo porcino. El tratamiento consistió en el lavado con agua fría y luego el desinfectado con una solución de monopersulfato de potasio modificado al 1% aplicado a través de un espumador de baja presión. El trailer no se secó. Resultados: En 10 de 150 muestras recolectadas de las 10 réplicas, se detectó el RNA del PRRSV 120 minutos después del tratamiento. La diferencia en los porcentajes de hisopos positivos a PCR recolectados a los 0 y 120 minutos después del tratamiento en las réplicas de control y tratamiento fue significativa (P < .001; prueba exacta de Fisher). No se detectó virus viable mediante la prueba de bioensayo porcino. Implicación: El lavado con alta presión de trailers de transporte, seguido de 120 minutos de exposición monopersulfato de potasio modificado al 1% aplicado con un hidroespumador, posiblemente eliminará el PRRSV infeccioso residual. | ResuméObjectif: Évaluer un protocole de désinfection des véhicules de transport commerciaux contaminés avec le virus du syndrome reproducteur et respiratoire porcin (PRRSV), en utilisant les conditions retrouvées sur les unités de production porcine commerciales. Ces conditions comprenaient l’utilisation d’eau froide pour le lavage (21°C), l’application d’un désinfectant commercial à l’aide d’un appareil moussant à basse pression, et la complétion du processus en ≤ 2 heures. Matériels et méthodes: Quinze sites à l’intérieur d’une remorque ont été contaminés expérimentalement avec le vaccin PRRS vivant modifié IngelVac (Boehringer Ingelheim Vetmedica Inc, St Joseph, Missouri; valeur médiane de 5 × 105 doses infectieuses de culture cellulaire par site). Dix réplications ont été faites. La présence d’ARN du PRRSV a été vérifiée par réaction d’amplification en chaîne par la polymérase (PCR) à partir d’écouvillonnages effectués à l’intérieur de la remorque avant le traitement et 120 minutes post-traitement. Les écouvillons positifs par PCR étaient par la suite testés pour la présence de PRRSV viable par un bio-essai utilisant des porcs. Le traitement de la remorque comprenait un lavage à l’eau froide et une désinfection avec une solution de 1% de monopersulfate de potassium modifié appliquée à l’aide d’un appareil moussant à basse pression. La remorque n’était pas asséchée. Résultats: De l’ARN du PRRSV a été détecté à 120 minutes post-traitement à partir de 10 des 150 échantillons recueillis lors des 10 réplications. Des différences significatives dans les pourcentages d’écouvillons positifs par PCR prélevés aux temps 0 et 120 minutes post-traitement ont été notées entre les réplications témoin négatif et traitement (P < .001; test exact de Fisher). Le bio-essai porcin n’a pas permis de détecter de virus vivant. Implication: Le lavage à haute-pression des remorques de transport, suivi d’une exposition pendant 120 minutes à une solution à 1% de monopersulfate de potassium modifié appliquée à l’aide d’un appareil moussant, permettrait fort probablement d’éliminer de ces remorques les PRRSV infectieux résiduels. |
Keywords: swine, disinfectant,
porcine reproductive and respiratory syndrome virus, transport vehicle
Search the AASV web site
for pages with similar keywords.
Received: September
18, 2005
Accepted: January
3, 2006
Porcine reproductive and respiratory syndrome virus (PRRSV), a single-stranded enveloped RNA virus classified in the order Nidovirales, family Arteriviridae, and genus Arterivirus,1 causes the condition known as porcine reproductive and respiratory syndrome (PRRS). This disease has proven to be very difficult to control consistently over time and across farms. One of the key components to successful control of PRRS is prevention of PRRSV transmission within and between farms. Transmission can occur through a number of reported routes, including infected pigs, semen, contaminated fomites, insects, avian species, and aerosols.2-8 Another potential route of PRRSV transmission between farms may be the livestock transport vehicle.9 In today’s modern pig industry, application of multi-site production technology has resulted in greater distances between sites and more movement of pigs between farms and to slaughter. Therefore, pig transport has become an important risk factor for the spread of PRRSV. In support of this hypothesis, previously published reports have demonstrated how motorized vehicles can mechanically transport PRRSV over distances of 50 km, and specific assessments of the role of the transport vehicle in the spread of PRRSV have been conducted.10-12 In one study,12 scale models (1:150) of weaned pig trailers were used to enhance study power. These models used materials and designs similar to those of commercial transport vehicles, and provided an animal density equal to that of a full-size trailer capable of transporting 300 weaned pigs. Under the conditions of that study, it was demonstrated that PRRSV-naive swine could become infected with PRRSV through contact with the contaminated interior of the transport models, that the concentration of PRRSV required to infect naive sentinel pigs was 1 × 103 median tissue culture infectious doses (TCID50), and that allowing the trailer to dry for 8 hours after washing prevented infection in 10 of 10 replicates.12
However, discussion of these results with veterinarians working in large commercial systems indicated that sanitation programs requiring time periods > 2 hours limit the cost-effective use of trailers. Furthermore, accessibility of hot water for washing (80°C) was limited, and use of a low-pressure foaming system was a common method of applying disinfectant. The use of foam provided an effective vehicle to carry the disinfectant to the target surface and a means to hold it there in the short term. This technique has the added advantage that it allows the operator to see where the disinfectant has been applied.
Despite the growing interest in the use of foaming as a technique to apply disinfectants, there was little scientific evidence demonstrating its efficacy against PRRSV. Recently, the foaming technique was used to test the efficacy of 1% modified potassium monopersulfate in scale models of weaned pig trailers experimentally contaminated with PRRSV.13 The results indicated that 120 minutes exposure to modified potassium monopersulfate applied with a hydrofoamer will most likely eliminate residual infectious PRRSV. However, while the results were interesting, an acknowledged limitation of this study was the use of trailer models to test the efficacy of this protocol instead of actual transport vehicles. Therefore, the objective of this study was to test the protocol in a full-size livestock trailer experimentally contaminated with PRRSV.
Materials and methods
Description of trailer
For this objective, an aluminum livestock trailer was employed (EBY Livestock Trailers, Blue Ball, Pennsylvania). The trailer was 18 m in length and 2.7 m in both height and width and consisted of an upper and lower level (double-decked). A loading ramp consisting of 11 steps was fastened inside the trailer to facilitate animal movement between the upper and lower levels. Each level contained three hinged gates that could be used to divide the area into equal-sized pens. During the study, the trailer was housed outdoors at the University of Minnesota Swine Disease Eradication Center research farm in west central Minnesota during the month of August 2005. To facilitate drainage post washing, the trailer was parked on a hill, allowing for a 3% slope.
Trailer contamination protocol
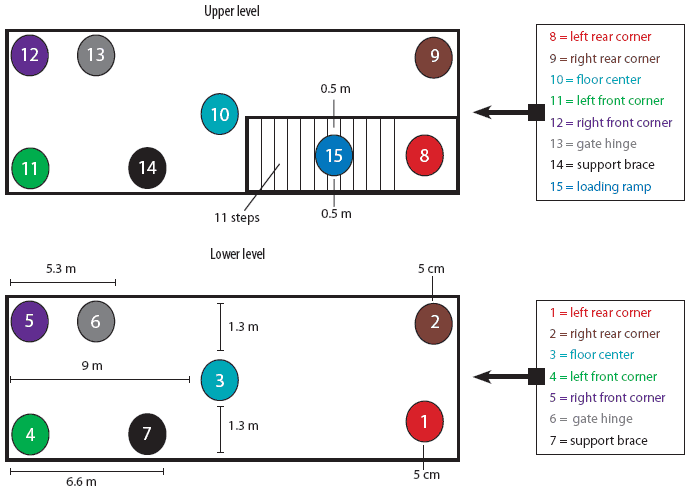
For the purpose of PRRSV contamination of the trailer, a specific protocol was employed. This protocol consisted of first a 10-minute wash using a commercial power washer (American Made Cleaners, Beresford, South Dakota) capable of delivering cold water (21°C) at 10,500 kPa, until all surfaces were visibly wet. Then 5-mL aliquots of IngelVac PRRS MLV vaccine (Boehringer Ingelheim Vetmedica, St Joseph, Missouri; 5 × 105 TCID50 total dose) were applied to 15 different sites throughout the trailer (Figure 1) using a syringe. These sites included the left rear, right rear, left front, and right front corners on both the upper and lower levels, the middle of the floor on the lower and upper levels, the hinge on the gate closest to the front of the trailer, ceiling support braces on both levels, and the loading ramp. For inoculation of the corners on both levels, the aliquot of vaccine was placed on the floor 5 cm from the contributing sides of the corner. For inoculation of the middle of the floor, the aliquot of vaccine was placed on a point exactly 1.3 m from either side of the trailer and 9 m from the front and rear walls. The second gate hinge on each level was inoculated by dripping the 5 mL of vaccine inside the hinge. This hinge was located 5.3 m from the front end of the trailer and approximately 0.4 m off the floor. Each ceiling support brace was inoculated by expelling the 5 mL of vaccine onto the top of the brace, approximately 6.6 m from the front end of the trailer and 1 m off the floor. Finally, 5 mL of vaccine was placed on the middle of the loading ramp (step number six) approximately 0.5 m from either edge.
| Figure 1: Diagram of 15 sites selected for contamination
with porcine reproductive and respiratory syndrome virus in a full-size,
double-decked trailer used to transport pigs.
|
Selection and application of disinfectant
The disinfectant used in this investigation was a modified potassium monopersulfate (Virkon S; DuPont Animal Health Solutions, Stone Mountain, Georgia). This product was selected on the basis of commercial availability and degree of usage in pig production facilities and transport biosecurity programs throughout North America. Modified potassium monopersulfate disinfectants act by denaturing microorganism proteins and enzymes and increasing virus plasmid permeability by disrupting sulfhydryl (-SH) and sulfur (S-S) bonds, which causes cell lysis and exposure of nucleic acids.14-16 After the trailer had been washed and contaminated with PRRSV, a 1% solution of modified potassium monopersulfate was applied with a hydrofoamer (Hydro Systems Company, Cincinnati, Ohio) attached to a garden hose. All visible surfaces of the trailer interior were covered with foamed disinfectant. Study personnel blinded to the location of the 15 sites of PRRSV contamination applied the disinfectant at all times throughout the study.
Diagnostic monitoring
The 15 contaminated sites were swabbed immediately after trailer contamination and 120 minutes post treatment. Sterile Dacron swabs (Fisher Scientific, Hanover Park, Illinois) were applied to the point of PRRSV contamination, then stored in 1 mL of sterile saline and frozen at -20°C to inactivate the disinfectant and preserve PRRSV RNA, as previously described.17,18 After collection of all required samples, swabs were tested for PRRSV RNA by polymerase chain reaction (PCR) using the TaqMan PCR assay (Perkin-Elmer Applied Biosystems, Foster City, California).19
All PCR-positive swabs collected at 0 and 120 minutes post treatment were tested for infectious PRRSV by swine bioassay.20 One-mL aliquots of the PCR-positive samples collected at each time post treatment were pooled across the 10 replicates. For the swine bioassay, 10 mL of each pooled sample was administered by intramuscular injection to a PRRSV-naive pig (sentinel). Sentinels were housed individually in isolated rooms to prevent transmission between animals and were tested 7 and 14 days post inoculation by serum PCR and ELISA. During the course of the study, animals were cared for according to an approved protocol based on guidelines of the University of Minnesota Institutional Animal Care and Use Committee.
Protocols of biosecurity
After each replicate was completed, trailers were re-washed and dried with high velocity air (12.4 m per second) at 88°C to 92°C, applied to the trailer interior for a 2-hour period using a Chinook heater (MAC Inc, Glenburn, North Dakota) capable of generating 1.2 million BTU of heat per hour. After drying, the 15 sites were again swabbed and tested by PCR as described to verify that trailers were free of residual PRRSV RNA, so that the results from each replicate were independent events and were not artificially impacted by residual RNA.
Controls
On each experimental day, a negative and then a positive control replicate was conducted prior to application of treatment. For negative controls, all 15 sites were sham-inoculated with sterile saline, treated without the use of disinfectant, and sampled 2 hours later as described. For positive controls, the 15 sites were inoculated with vaccine as described, then sampled 2 hours later without application of any treatment. After the positive control was completed, the trailer was washed and dried as described for treatment replicates.
Data analysis
Differences in the percentages of PCR-positive swabs (the number of positive swabs divided by the total number of swabs collected) at 0 and 120 minutes post treatment in treated and control trailers were compared using Fisher’s exact test.
Results
This study was conducted over a 5-day period (two replicates per day). During that time, environmental temperature averaged 21.4°C (range, 17°C to 25°C) and relative humidity averaged 80% (range, 67% to 95%). A total of 150 swabs at each sampling point were collected across the 10 replicates (15 swabs per replicate).
At 0 minutes, 150 of 150 swabs (100%) were PCR-positive, and all pooled samples were bioassay-positive. At 120 minutes, 10 of 150 swabs (6.7%) were PCR-positive; however, all samples were swine bioassay-negative. The 10 PCR-positive swabs were obtained from a lower-level corner (n = 1), lower-level gates hinges (n = 4), upper-level ceiling braces (n = 3), and lower-level ceiling braces (n = 2). The difference in percentages of PCR-positive swabs collected at 0 minutes and 120 minutes was significant (P < .001). All samples collected from all 15 sites of all positive-control replicates (n = 5) at 0 minutes were PCR-positive. The number of PCR-positive samples collected from the positive-control replicates (n = 5) at 120 minutes ranged from five to 10 of the 15 sites (mean = 53%), and pooled samples were swine bioassay-positive at both sampling periods. All samples from negative-control replicates (n = 5) and all swabs collected from the dried trailer between replicates were PCR-negative.
Discussion
The objective of this study was to test a sanitation protocol designed for PRRSV-contaminated commercial livestock vehicles involving practices frequently utilized in large-scale commercial swine production systems. Specific practices incorporated in the study design were use of cold water for washing, application of a commercially available disinfectant by low-pressure foaming, and turn-around time = 2 hours. Scientific data on the efficacy of foaming for decontamination of PRRSV-positive transport were not available, and use of this method of application of disinfectant was rapidly increasing in many production systems in North America. A previous study used this same sanitation protocol in scale models of transport vehicles.13 The present results support previous data.13
This study contained several acknowledged limitations. First, it was not possible to counteract the impact of drying that naturally occurred during the sampling period of 120 minutes. Drying is highly efficacious for eliminating PRRSV from the interiors of contaminated trailers.12 It is also not known if the high concentration of PRRSV used to contaminate the trailers was representative of field conditions. It has been previously determined12 that sentinel pigs can be infected with PRRSV in model trailers contaminated with 1 × 103 TCID50 of PRRSV. Therefore, in order to aggressively test the efficacy of the decontamination protocol, a high concentration of virus was selected. Furthermore, although a relatively large number of replicates was conducted, this was insufficient to predict the frequency of the events recorded in the study. Also, the results of this study cannot be directly extrapolated to other swine pathogens, such as transmissible gastroenteritis virus or Escherichia coli. The study design did not include debris (eg, fecal material, bedding) in the trailer as is typically encountered under commercial swine production conditions, and it is unknown whether the presence of such material would have affected the outcome. However, we chose to start with a debris-free, wet trailer to evaluate the efficacy of the protocol under these conditions. Further studies designed to test the impact of debris may be helpful. Finally, due to this approach, we did not use detergents to facilitate removal of debris, and the inclusion of such products might have enhanced the results and decreased the time required for cleaning.
Despite its limitations, the study had considerable strength. It included use of a full-size transport vehicle, a hydrofoamer, positive and negative control replicates, and study personnel who were blinded to the location of the PRRSV inoculation sites during the application of the disinfectant. The hydrofoamer is easy to use, and its ability to provide visual confirmation of contact between the disinfectant and the surface (ie, white foam) ensures better and more accurate application of disinfectant in repeated commercial usage. Also, through the use of multiple diagnostic methods, this study showed that the modified potassium monopersulfate product tested produced good inactivation of PRRSV within the target time when cold water was used and disinfectant was applied by foaming. During the process of sample handling, special care was taken to minimize the possibility of degradation of PRRSV RNA in swab samples secondary to prolonged contact with disinfectant during storage. Because it was not possible to add a compound to the sample to neutralize the disinfectant, swabs were stored immediately post collection at -20°C to retard disinfectant activity,14-16 and underwent RNA extraction within 24 hours post collection, the standard practice in previous similar studies.17-18
An interesting observation made during the study was the location of the PCR-positive samples at 120 minutes post treatment. The rationale behind selection of the 15 specific sites in the trailer was inclusion of a subset of sites which, depending on the design of the trailer, may contain physical impediments to the treatment process, eg, gate hinge, ceiling brace, corners. The remaining sites were selected on the basis of expected ease of treatment, for example, the middle of the floor. Finally, a double-decked trailer was desired because of the frequency of its use in the industry and speculation that it may be more difficult to sanitize than a single-deck unit. Numerically more positive samples were detected in front corners on the lower levels, and gate hinges and braces on both levels, while no positive samples were detected on the middle of the floor. Yet despite the presence of PRRSV RNA in these sites, the number of positive samples was significantly reduced after 120 minutes, and swine bioassay verified the absence of viable virus at 120 minutes in all 10 replicates. Therefore, it may be advisable for swine producers to pay close attention to certain sites when disinfecting trailers.
Implication
- High-pressure washing of transport trailers, followed by 120 minutes exposure to 1% modified potassium monopersulfate applied with a hydrofoamer, will most likely eliminate residual infectious PRRSV.
Acknowledgements
The authors would like to thank the National Pork Board for financial support for this study and PIC for use of the trailer.
References
1. Cavanagh N. Nidovirales. A new order comprising Coronoviridae and Arteriviridae. Arch Virol. 1997;142:629–633.
2. Dee SA, Yoon HS, Pijoan C. Controlling the spread of PRRS virus in the breeding herd through management of the gilt pool. Swine Health Prod. 1994;3:64–69.
3. Christopher-Hennings J, Nelson EA, Hines RJ, Nelson JK, Swenson SL, Zimmerman JJ, Chase CCL, Yaeger MJ, Benfield DA. Persistence of porcine reproductive and respiratory syndrome virus in serum and semen of adult boars. J Vet Diagn Invest. 1995;7:456–464.
4. Otake S, Dee SA, Rossow KD, Joo HS, Deen J, Molitor TW. Transmission of porcine reproductive and respiratory syndrome virus by fomites. J Swine Health Prod. 2002;10:59–65.
5. Otake S, Dee SA, Rossow KD, Moon RD, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by mosquitoes. Can J Vet Res. 2002;66:191–195.
6. Otake S, Dee SA, Rossow KD, Moon RD, Trincado C, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by houseflies (Musca domestica). Vet Rec. 2003;152:73–76.
7. Zimmerman JJ, Yoon KJ, Pirtle FC, Wills RW, Sanderson TJ, McGinley MJ. Studies of porcine reproductive and respiratory syndrome virus in avian species. Vet Microbiol. 1997;55:329–336.
8. Torremorell M, Pijoan C, Janni K, Walker R, Joo HS. Airborne transmission of Actinobacillus pleuropneumoniae and porcine reproductive and respiratory syndrome virus in nursery pigs. Am J Vet Res. 1997;58:828–832.
9. Poumian E. Disinfection of trucks and trailers. Rev Sci Tech Off Int Epiz. 1995;14:171–176.
10. Dee SA, Deen J, Rossow KD, Mahlum C, Otake S, Joo HS, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during cold weather. Can J Vet Res. 2002;66:232–239.
11. Dee SA, Deen J, Rossow KD, Mahlum C, Eliason R, Otake S, Joo HS, Pijoan C. Mechanical transmission of porcine reproductive and respiratory syndrome virus throughout a coordinated sequence of events during warm weather. Can J Vet Res. 2003;67:12–16.
12. Dee SA, Deen J, Otake S, Pijoan C. An experimental model to evaluate the role of transport vehicles as a source of porcine reproductive and respiratory syndrome virus to susceptible pigs. Can J Vet Res. 2004;68:128–133.
13. Dee SA, Deen J. Evaluation of an industry-based sanitation protocol for transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. J Swine Health Prod. 2006;14:126–132.
14. Russell AD, Hugo WB. Chemical disinfectants. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. 1st ed. Oxford, England: Blackwell Scientific Publications Ltd; 1987:12–42.
15. Quinn PJ. Evaluation of veterinary disinfectant and disinfection processes. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. 1st ed. Oxford, England: Blackwell Scientific Publications Ltd; 1987:66–116.
16. Morgan-Jones S. Practical aspects of disinfection and infection control. In: Linton AH, Hugo WB, Russell AD, eds. Disinfection in Veterinary and Farm Animal Practice. 1st ed. Oxford, England: Blackwell Scientific Publications Ltd; 1987:144–167.
17. Dee SA, Deen J, Burns D, Douthit G, Pijoan C. An assessment of sanitation protocols for commercial transport vehicles contaminated with porcine reproductive and respiratory syndrome virus. Can J Vet Res. 2004;68:208–214.
18. Dee SA, Deen J, Pijoan C. Evaluation of disinfectants for the sanitation of porcine reproductive and respiratory syndrome virus-contaminated transport vehicles at cold temperatures. Can J Vet Res. 2005;69:64–70.
*19. Molitor TW, Tune KA, Shin J, Collins J, Kapur V. Applications of TaqMan™ PCR in the detection of porcine reproductive and respiratory syndrome virus. Proc AD Leman Swine Conf. Brooklyn Center, Minnesota. 1997;173–175.
20. Swenson SI, Hill HT, Zimmerman JJ, Evans LE, Landgraf JG, Wills RW, McGinely M, Brevik AK, Ciszewski D, Frey ML. Excretion of porcine reproductive and respiratory syndrome virus after experimentally induced infection in boars. JAVMA. 1994;204:1943–1948.
* Non-refereed reference.