Original research |
Peer reviewed |
Association between umbilical hernias and genetic line in a swine multiplication herd and methods to differentiate the role of sire in the incidence of umbilical hernias in offspring
Asociación entre hernias umbilicales y línea genética en una piara multiplicadora porcina y métodos para diferenciar el papel del semental en la incidencia de hernias umbilicales en las crías
Association entre la présence d’hernies ombilicales et la lignée génétique à l’intérieur d’un troupeau multiplicateur de porcs et méthodes pour différencier le rôle des mâles dans l’incidence d’hernies ombilicales chez les rejetons
Stephanie C. Rutten-Ramos, DVM; John Deen, DVM, MSc, PhD, Diplomate ABVP
College of Veterinary Medicine, Department of Clinical and Population Sciences, University of Minnesota, St Paul, Minnesota. Corresponding author: Dr Stephanie C. Rutten-Ramos, University of Minnesota, 385 AnSci/VetMed, 1988 Fitch Avenue, St Paul, MN 55108; Tel: 612-624-7762; Fax: 612-625-1210; E-mail: rutt0011@umn.edu.
Cite as: Rutten-Ramos SC, Deen J. Association between umbilical hernias and genetic line in a swine multiplication herd and methods to differentiate the role of sire in the incidence of umbilical hernias in offspring. J Swine Health Prod. 2006;14(6):317–322.
Also available as a PDF.
SummaryObjectives: To determine the existence of a link between genetic line and incidence of umbilical hernias in nursery pigs and whether this incidence differs among sires, and to develop a model to identify sires with a high incidence of umbilical hernias among offspring. Materials and methods: Gilt and boar progeny from 8276 litters of a genetic multiplier that used four dam lines and five sire lines were observed for umbilical hernias by 11 weeks of age. Hernias were attributed to birth litter. Odds of umbilical hernia development were calculated using logistic regression and rates were calculated using Poisson regression. Negative binomial models using sire as a random effect were used to predict incidence of hernias and hernia-positive litters from maternal-line sires with ≥ 25 single-sire litters. Results: Odds of umbilical hernia-positive litters were different among sire and progeny lines (P < .01). Rates of umbilical hernias were significantly different between genetic lines. The rate of umbilical hernias in pure maternal-line products was nearly twice that in out-crossed lines (P < .001). For individual-sire predicted hernias compared to observed umbilical hernias, R2 was 0.960, and for individual-sire predicted hernias per litter compared to observed hernias per litter, R2 was 0.816. Implications: Umbilical hernias may be influenced by a genetic component. Progeny testing using 25 single-sire litters identifies potentially heritable defects that occur at a rate twice that in the normal population. Negative binomial models can effectively predict rates of event occurrence. | ResumenObjetivos: Determinar la existencia de una relación entre línea genética e incidencia de hernias umbilicales en cerdos destetados y si esta incidencia difiere entre sementales, y desarrollar un modelo para identificar machos con una alta incidencia de hernias umbilicales entre las crías. Materiales y métodos: Se observaron hembras y machos descendientes de 8276 camadas de un multiplicador genético que utilizó cuatro líneas de hembras y cinco líneas de machos en busca de hernias umbilicales a las 11 semanas de edad. Las hernias se atribuyeron a la camada de nacimiento. La probabilidad de desarrollo de hernia umbilical se calculó utilizando regresión logística y los porcentajes se calcularon utilizando la regresión de Poisson. Se utilizaron modelos binominales negativos utilizando al semental como efecto al azar para predecir la incidencia de hernias y camadas positivas a hernias de sementales de línea materna con ≥ 25 camadas de un solo semental. Resultados: La posibilidad de camadas positivas a la presencia de hernia umbilical fueron diferentes entre sementales y líneas (P < .01). El porcentaje de hernias umbilicales fue significativamente diferente entre líneas genéticas. El índice de hernias umbilicales en productos de línea materna pura fue casi el doble que en líneas híbridas (P < .001). Para las hernias pronosticadas por semental de manera individual comparadas con hernias umbilicales observadas, el R2 fue de 0.960, y para las hernias pronosticadas por semental individual por camada comparada con hernias observadas por camada de un semental, el R2 fue 0.816. Implicaciones: Las hernias umbilicales pueden ser influidas por un componente genético. La prueba de descendencia utilizando 25 camadas de un solo semental identifica potenciales defectos heredables que ocurren en un índice del doble que en la población normal. Los modelos binominales negativos pueden pronosticar eficazmente los índices de ocurrencia del evento.
| ResuméObjectifs: Déterminer l’existence d’un lien entre
la lignée génétique et l’incidence d’hernies
ombilicales chez des porcs en pouponnière et vérifier si l’incidence
diffère en fonction du mâle; développer un modèle
pour identifier les mâles ayant une incidence élevée d’hernie
ombilicale parmi leurs rejetons. Résultats: Les probabilités d’obtenir des portées avec hernie ombilicale étaient différentes parmi les verrats et les lignées de progéniture (P < .01). Les taux d’hernies ombilicales étaient significativement différents entre les lignées génétiques. Le taux d’hernies ombilicales chez les produits de lignée maternelle pure était près du double de celui des lignées croisées (P < .001). Une valeur R2 de 0.960 a été obtenue lorsque l’on a comparé le nombre prédit d’hernies au nombre observé d’hernies ombilicales pour les verrats pris individuellement, de même qu’une valeur R2 de 0.816 a été obtenue lorsque l’on a comparé le nombre prédit d’hernies par portée et le nombre observé d’hernies par portée pour un verrat. Implications: La présence d’hernies ombilicales peut être influencée par une composante génétique. Des tests de progéniture sur 25 portées à géniteur mâle unique permettent d’identifier des défauts potentiellement héritables qui se produisent deux fois plus fréquemment que dans la population normale. Les modèles binomiaux négatifs permettent de prédire les taux d’occurrence d’un évènement. |
Keywords: swine, umbilical
hernia, genetic line, sire
Search the AASV web site
for pages with similar keywords.
Received: September
1, 2005
Accepted: February
13, 2006
Umbilical hernias are common in swine.1 Though their cause is not well defined, perinatal umbilical infections, dystocia, navel sucking, and genetic components may contribute to their occurrence.2 In 1994, Searcy-Bernal et al3 reported that most hernias appear in pigs 9 to 14 weeks of age. Hernia occurrence differed between genetic lines, and prophylactic antibiotic use at birth did not show a protective effect. Additionally, qualitative assessment of omphalitis (external inflammation at the umbilicus) at weaning was not associated with subsequent hernia development.3 Two previous studies attributed high rates of umbilical hernias to individual boars.1,3 Warwick1 reported that elimination of two sires from a research herd reduced umbilical hernia prevalence by 50% among boars raised to 1 month of age.
In other species, genetic components have been identified as causes of umbilical hernias and associated disease. Angus and Young4 reported two sire-associated cases of umbilical hernias in offspring of different cattle breeds. Abnormal urachal structures have been associated with masses at the umbilicus and with umbilical hernias.5-10 Borras5 reported persistent urachus and abscess development in two colonies of Wistar rats. In human cases, purulent urachal cysts are believed to have become infected via a communication with the bladder,9 and a familial case of urachal cysts in humans has also been described.10
Angus and Young4 hypothesized that several genes are involved in the formation of umbilical hernias. Mode of gene inheritance would affect appearance of the defect in the offspring. Dominant traits and dominant traits with low penetration would be expected to appear in the first generation, while recessive traits would not appear until the genes have been more widely distributed in the population. Use of artificial insemination has the potential to allow widespread dissemination of undesirable traits before they are detected, because of the ability to use a single sire across many females.4
Maximum likelihood functions can be used to predict expectations for long-range performance of individuals. Fixed and random effects can be incorporated to create mixed models.11 In animal applications of mixed models, fixed effects include variables such as breed, which apply to all individuals. The random effect applies to a sample of the fixed effect, such as the individual sire. By their nature, models incorporating random effects feature “shrinkage”; that is, the predictions generated for the individuals are all drawn toward the population mean. The effect, then, is to smooth the individual estimates by effectively using a larger sample size.11
Several types of mixed models exist. Poisson models are often used to handle count data, or data with a fixed possibility of events. Count data are prone to overdispersion; that is, the data’s variance is greater than the mean.11 Negative binomial models have the ability to handle fixed and random effects for populations with Poisson distributions and are therefore useful in predicting long-range expectations for the number of events expected to occur.11
This study was conducted in two phases. In the first phase (Phase One), the objectives of the study were to determine if umbilical hernias occurring in a swine herd were associated with genetic line and to estimate the effect of genetic line on the development of umbilical hernias. Given evidence of a genetic-line association, the objectives of the second phase (Phase Two) were to determine if the prevalence of umbilical hernia occurrence in a swine herd differs among sires and to develop a model to efficiently identify sires with high incidence of umbilical hernias in their offspring. The study is reported as part of an observational investigation of umbilical hernias in a sow herd maintaining several genetic lines.
Materials and methods
Study population
Subjects were born over a 16-month time interval in a 2800-sow herd with four dam lines and five sire lines (Table 1). Of these, three dam lines were used for maternal production and one for paternal production. Line-by-line combinations were created according to a specified genetic protocol (Figure 1), and gilt and boar progeny were assigned litter-specific identification at birth. Replacement females for the sow herd were derived both internally and from another system containing the same genetic lines. Matings were all performed via artificial insemination using semen from two studs containing the same genetic lines. All semen was homospermic. For the last 10 months of the study period, the unit discontinued clipping dried navel cords, and during the last 7 months of the study period, long-acting ceftiofur was administered to all piglets at birth. The herd was free of Mycoplasma hyopneumoniae and porcine reproductive and respiratory syndrome virus. The herd was managed with due regard for animal welfare considerations.
Table 1: Definition of abbreviations describing genetic lines in a 2800-sow herd
|
Figure 1: Mating protocol in a 2800-sow herd in which gilt and boar progeny in the nursery were evaluated once between 7 and 11 weeks of age for umbilical hernias. Abbreviations describing genetic lines are shown in Table 1. Maternal lines were used to generate replacement gilts, and paternal lines were used to produce replacement boars. Progeny observed corresponded with the type of replacement produced. 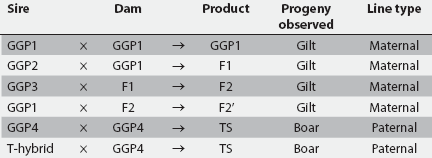
|
Data collection and compilation
All breeding herd information was maintained in a PigCHAMP database (PigCHAMP, Inc, Ames, Iowa). Litter information for all litters born within the 16-month period, including dam, dam line, sire, sire line, farrowing date, and litter ID, were maintained in an Excel spreadsheet (Microsoft Corporation, Redmond, Washington). While in the nursery, each group of gilt and boar progeny was evaluated for umbilical hernias one time between 7 and 11 weeks of age. Barrow progeny from maternal lines and gilt progeny from paternal lines were not included in this study because half of those animals left the system at weaning. Identification of herniated animals was recorded and attributed to the birth litter. Incomplete, illegible, or nonsensical identification was omitted.
Data analysis
Phase One. All analyses were performed in SAS version 9.1 (SAS Institute, Inc, Cary, North Carolina). Because of small litter numbers and genetic similarity, the two sire lines used to produce boar progeny from a common dam line were combined into a single category to be used as the reference. Logistic regression analysis was used to determine the odds of identifying at least one hernia in the gilt or boar offspring of a litter. Log-linear evaluations were conducted for sire line, sire line and dam line, and product line. Poisson regression was used to determine the rate of hernia identification per litter by sire line and product line.
Phase Two. All data analyses were performed in SAS version 9.1. All maternal line sires with = 25 single-sire litters were included. This was selected as the minimum number for inclusion in order to identify boars with twice the normal rate of umbilical hernia occurrence with 95% confidence (a = 0.05 andß = 0.8). Litters were stand-ardized by log transformation by all sires and by sire line, and data were sorted by the total number of hernias observed. Negative binomial models incorporating sire as a random effect were evaluated using line-specific population regressions and a single regression for the entire population and with litters standardized by line and by all sires. The best regression approach was selected on the basis of fit statistics (Akaike’s Information Criterion).12 Negative binomial models were then used to estimate both the total number of umbilical hernias expected per sire and the number of litters per sire in which it was expected that at least one umbilical hernia would be observed (hernia-positive litters). Sire was treated as a random effect in both models (Figure 2). Correlations between observed and estimated values were calculated and the models reviewed for their assumptions. Observed total hernias, hernia-positive litters, and their respective estimates were each divided by total litters per sire to determine the long-run expectation of hernia incidence per sire and hernia-positive litters per sire.
| Figure 2: This negative binomial model was used
to predict the number of umbilical hernias observed from a given sire in
a 2800-sow herd with four dam lines and five sire lines. Progeny were observed
for umbilical hernias once in the nursery between 7 and 11 weeks of age.
|
Results
Phase One. A total of 8276 litters were considered in the analyses. The number of litters by sire and dam line combinations is shown in Table 2. No significant effects on hernia prevalence were observed after navel-cord clipping was discontinued or after administration of long-acting ceftiofur to piglets at birth was initiated. The odds of identifying at least one hernia in a litter of observed product offspring were different from the reference line for each of the remaining sire, dam, and product lines (P < .01). The odds of identifying at least one hernia in select product offspring of a GGP1 litter were nearly twice that of all other maternal products and 50 times that of the reference line (Table 3). Use of the GGP1 dam increased the odds of at least one hernia in the litter by 1.8 (P < .001).
Table 2: Study litters observed for umbilical hernias by sire line × dam line* combinations
* Abbreviations defined in Table 1. NA = not applicable. |
||||||||||||||||||||||||||||||||||||||||||
Table 3: Odds of identifying at least one hernia in observed progeny from a total of 8276 litters observed once for umbilical hernias between 7 and 11 weeks of age
* Abbreviations defined in Table 1. † OR = odds ratio derived from logistic regression. ‡ P values reflect chi-square values for the logistic regression estimates. NA = not applicable. |
The Poisson estimates for sire and product lines were significant (P < .001), and rates of hernia identification among select products of a litter differed between sire and product lines (Table 4).
Table 4: Rate estimates*of umbilical hernias by progeny line among 8276 litters observed for hernias once in the nursery between 7 and 11 weeks of age
* Rate of umbilical hernias observed per 1000 litters estimated using Poisson regression. † Abbreviations defined in Table 1. ‡ P values reflect chi-square values for the Poisson regression estimates. NA = not applicable. |
Phase Two. A total of 32 sires and 1823 litters were included in the analysis (range, 26 to 121 litters per sire). The best-fitting models used a single regression line for the entire population with litters standardized by genetic line. Hernias were identified in pigs from 209 litters (11.5% of litters). Table 5 lists the sire estimates for hernias identified per litter and the percent of litters identified with hernias. For predicted versus observed umbilical hernias, R2 was 0.960, and for predicted versus observed umbilical hernia-positive litters, R2 was 0.914. For predicted versus observed umbilical hernias per litter, R2 was 0.816, and for predicted versus observed umbilical hernia-positive litters per litter, R2 was 0.592. Observed values fell within the 95% confidence limits for all estimates.
Table 5: Umbilical hernias identified in gilt offspring observed once between 7 and 11 weeks of age and sire estimates for hernias identified per litter and for percent of litters identified with hernias
* Abbreviations defined in Table 1. † Observed rates were calculated as (number of umbilical hernias ÷ number of litters observed) × 100 and (number of umbilical hernia-positive litters ÷ number of litters observed) × 100, respectively, for each sire. ‡ Predicted rates were calculated as (model estimate of umbilical hernias ÷ number litters observed) × 100 and (model estimate of umbilical hernia-positive litters ÷ number litters observed) × 100, respectively, for each sire. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
The observational nature of this study limited its ability to completely describe the extent of the problem. Litters were observed over a 16-month period. To accommodate system flow and labor, hernias were identified every other week among nursery pigs 7 to 11 weeks of age, before they left the system. Since most hernias appear by 9 to 14 weeks of age,3 this data set probably underrepresented the total number of hernias in the population. Additionally, approximately 5% of the herniated animals had illegible tattoos at the time of evaluation, and although their lineage was known, they were omitted from the data set because they could not be assigned to a litter. Among evaluated lines and sires, litter, instead of boars or gilts born, was used as the denominator, since identification of animals that died was not recorded.
Additionally, because of the unit’s mating program, the effect of dam line on occurrence of umbilical hernias could not be effectively measured. Only the GGP1 dam line was measured across two sire lines. Influence of the F1 and F2 dam lines could not be measured, and since the F1 and F2 dams are 50% and 25% GGP1, their use may have overestimated the true effect of the GGP2 and GGP3 boar lines and sires. Furthermore, if the GGP2 and GGP3 lines have a predisposition to umbilical hernias, involvement of multiple genes does not favor the same defect or mode of inheritance in each of the lines.4 Additionally, it is possible that the age of hernia appearance differs among the genetic lines. However, on the basis of the analyses, including the role of the GGP1 in the occurrence of umbilical hernias, it is our conclusion that the GGP1 line contains a heritable defect, and appearance of umbilical hernias in the other lines is likely the result of hybrid combinations involving the GGP1.
Although the Phase One analyses generated significant P values, the inherent variability of the data set resulted in large confidence intervals. Even so, the lower bounds of the confidence intervals represent significant association between hernia occurrence and genetic background.
Only single-sire litters were used in the Phase Two analyses. The negative binomial model used is a shrinkage function; therefore, all estimates, including outliers, are pulled towards the population mean.11 Consequently, potential exists to overestimate defect rates in sires with low rates and underestimate rates in those with high occurrence. Though the estimates generated significant P values, the overdispersion of the data set resulted in wide confidence intervals.
As in the Phase One analyses, the inability to quantify the dam effect likely contributed to the ability of different models to fit the data. It is likely that the single regression line for the sire population was better than line-specific regressions because the dam effect was not quantified, and the different dam lines used across the evaluated sire lines are inherently related to each other. However, single-line regression models overestimated umbilical hernia occurrence for genetic lines with low hernia incidence. While the models with litters standardized for all sires generated narrower confidence intervals, their estimates were considerably less precise than those from models with litters standardized by genetic line. This is likely due to the previously observed discrepancy in hernia occurrence by genetic line.8 Because of the observational nature of this investigation and the use of a negative binomial model, estimates of heritability were not made.
Implications
- Umbilical hernias may be associated with genetic lineage.
- Progeny testing using 25 single-sire litters can identify potentially heritable defects that occur at a rate twice that in the normal population.
- Negative binomial models can effectively predict rates of event occurrence.
References
*1. Warwick BL. A study of hernia in swine. Bulletin 69. Madison, Wisconsin: Wisconsin Agriculture Experiment Station. 1926;1–22.
2. Ingwersen W. Digestive system: Congenital and inherited anomalies. In: Aiello SE, ed. The Merck Veterinary Manual, 8th ed. Whitehouse Station, New Jersey: Merck & Co, Inc; 1998:125–130.
3. Searcy-Bernal R, Gardner IA, Hird DW. Effects of and factors associated with umbilical hernias in a swine herd. JAVMA. 1994;204:1660–1664.
4. Angus K, Young GB. A note on the genetics of umbilical hernia. Vet Rec. 1972;90:245–247.
5. Borras M. Cases of persistent urachus with umbilical abscess in Wistar rats. Lab Anim. 1983;17:55–58.
6. Begg RC. The urachus: its anatomy, histology and development. J Anat. 1930;64:170–183.
7. Freer JA. Abnormalities of the urachus. Ann Surg. 1887;5:107–119.
8. MacMillan RW, Schullinger JN, Santulli TV. Pyourachus: an unusual surgical problem. J Ped Surg. 1973;8:387–389.
9. Brodie N. Infected urachal cysts. Amer J Surg. 1945;69:243–248.
10. Kubota K, Nomura S, Kawahara M, Kaminishi M. Familial urachal sinus associated with a possible congenital malformation: report of a case. Surg Today. 2003;33:237–239.
11. Agresti A, Booth JG, Hobert JP, Caffo B. Random-effects modeling of categorical response data. Sociol Methodol. 2000;30(1):27–80.
12. Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS® System for Mixed Models. Cary, North Carolina: SAS Institute, Inc; 1986:101-102.
*Non-refereed reference.

