Original research |
Peer reviewed |
Influence of gestation housing on sow behavior and fertility
Influencia de las instalaciones de gestación en el comportamiento y fertilidad de las hembras
Influence de l’hébergement durant la gestation sur le comportement et la fertilité des truies
Jarno Jansen; Roy N. Kirkwood, DVM, PhD, Diplomate ECAR; Adroaldo J. Zanella, DVM, PhD; Robert J. Tempelman, PhD
JJ, AJZ, RJT: Department of Animal Science, Michigan State University, East Lansing, Michigan. RNK, AJZ: Department of Large Animal Clinical Sciences, Michigan State University, East Lansing, Michigan. Dr. Zanella is now with the Norwegian School of Veterinary Science, Oslo, Norway. Corresponding author: Dr Roy Kirkwood, Department of Large Animal Clinical Sciences, Michigan State University, East Lansing, MI 48824-1314; Tel: 517-432-5198; Fax: 517-432-1042; E-mail: kirkwood@cvm.msu.edu.
Cite as: Jansen J, Kirkwood RN, Zanella AJ, et al. Influence of gestation housing on sow behavior and fertility. J Swine Health Prod. 2007;15(3):132–136.
Also available as a PDF.
SummaryObjective: To examine the effect of group housing sows on their behavior and fertility. Materials and methods: In Experiment One, at 65 to 70 days of gestation, 96 sows were assigned by parity to individual or group housing and observed for aggressive encounters during three 1.5-hour time blocks immediately after relocation and 1 day later. On the third day, feeding-time aggression was observed during two 30-minute feeding periods, starting when feed was dropped. Saliva samples obtained from unrestrained sows 1 day before and after relocation were assayed for cortisol concentrations. In Experiment Two, 937 mixed-parity sows in 10 weekly breeding groups were either housed in groups of approximately 50 (n = 462) or individually housed in gestation stalls (n = 475). For 140 individually-housed and 330 group-housed sows, backfat depths at the P2 position were determined using A-mode ultrasonography at gestation days 55 to 60, at farrowing, and at weaning. Results: Group-housed sows were involved in more aggressive encounters than stall-housed animals (P < .05). Aggressive encounters per hour were more numerous in grouped sows during feeding on day 3 than during the day of grouping (P < .001). Salivary cortisol concentrations were higher in grouped sows, but differences between pre-and post-relocation concentrations were not correlated with levels of aggression. There was no effect of housing on backfat depths or sow fertility. Implication: If sows are grouped during gestation, particular attention should be directed toward feeding management to avoid excessive aggression and possible adverse effects on welfare. | ResumenObjetivo: Examinar el efecto del alojamiento de hembras en grupo en su comportamiento y fertilidad. Materiales y métodos: En el Experimento Uno, de los 65 a los 70 días de gestación, 96 hembras fueron asignadas por parto a alojamiento individual o en grupo y se observaron los encuentros agresivos durante tres bloques de tiempo de 1.5 horas inmediatamente después de la reubicación y 1 día después. En el tercer día, se observó la agresión a la hora de la alimentación durante dos periodos de alimentación de 30 minutos, iniciando cuando se dejaba caer el alimento. Las muestras de saliva obtenidas de hembras libres 1 día antes y después de la reubicación se probaron en busca de concentraciones de cortisol. En el Experimento Dos, 937 hembras de paridad mixta en 10 grupos semanales de gestación fueron alojadas en grupos de aproximadamente 50 (n = 462) o alojadas individualmente en corrales de gestación (n = 475). En 140 hembras alojadas individualmente y las 330 alojadas en grupo, la profundidad de la grasa dorsal en la posición P2 se determinó utilizando ultrasonografía de modo A de los 55 a 60 días de gestación, al parto, y al destete. Resultados: Las hembras alojadas en grupo se involucraron en encuentros más agresivos que los animales alojados en corrales (P < .05). Los encuentros agresivos por hora fueron más numerosos en las hembras en grupo durante la alimentación en el día 3 que en el día de la agrupación (P < .001). Las concentraciones salivares de cortisol fueron más altas en las hembras alojadas en grupos, pero las diferencias entre las concentraciones pre y post reubicación no se correlacionaron con niveles de agresión. El alojamiento no tuvo efecto en la profundidad de la grasa dorsal o en la fertilidad de las hembras. Implicacion: Si las hembras se agrupan durante la gestación, se debe poner atención especial en el manejo de la alimentación para evitar agresión excesiva y posibles efectos adversos en su bienestar. | ResuméObjectif: Examiner l’effet de l’hébergement en groupe des truies sur leur comportement et leur fertilité. Matériels et méthodes: Au cours de l’Expérience 1, 96 truies de 65 à 70 jours en gestation ont été assignées par parité à un hébergement individuel ou en groupe et observées pour des rencontres agressives au cours de trois blocs de 1.5 heures immédiatement après leur relocalisation et 1 journée plus tard. Au troisième jour, la présence d’un comportement agressif lors du repas était notée durant deux périodes d’alimentation de 30 minutes, débutant lorsque la nourriture était distribuée. Les concentrations de cortisol dans des échantillons de salive obtenus de truies non contentionnées 1 jour avant et 1 jour après la relocalisation ont été mesurées. Au cours de l’Expérience 2, 937 truies de parités variées distribuées dans 10 groupes d’accouplement hebdomadaire étaient logées soit en groupe d’environ 50 (n = 462) ou individuellement dans des cages de maternité (n = 475). L’épaisseur du gras dorsal à la position P2 était mesurée à l’aide d’un appareil à ultrason en mode A pour 140 truies logées individuellement et 330 truies logées en groupe entre le 55e et 60e jour de gestation, lors de la mise-bas, et lors du sevrage. Résultats: Les truies logées en groupe étaient impliquées dans plus de rencontres agressives que les truies logées dans des cages de maternité (P < .05). Le nombre de rencontre agressive par heure était plus élevé, pour les truies regroupées, lors des repas au jour 3 que durant la journée du regroupement (P < .001). Les concentrations de cortisol salivaire étaient plus élevées chez les truies regroupées, mais les différences entre les concentrations pré- et post-relocalisation n’étaient pas corrélées avec les niveaux d’agression. Aucun effet n’a été noté entre le type d’hébergement et l’épaisseur du gras dorsal et la fertilité des truies. Implication: Si les truies sont regroupées durant la gestation, une attention particulière devrait être portée à la gestion des repas afin d’éviter des comportements agressifs excessifs et des effets néfastes possibles sur le bien-être des animaux. |
Keywords: swine, groups,
behavior, cortisol, fertility
Search the AASV web site
for pages with similar keywords.
Received: July
20, 2005
Accepted: July
17, 2006
The primary objective of the breeding herd is to maximize the number of pigs weaned per sow per year cost effectively, although, in the future, it is likely that consumer demands for perceived improved animal welfare must also be considered.1 The most common housing system for weaned and gestating sows in North America is the gestation stall, which allows for ease of individual feeding and artificial insemination. However, there is a perception that sow gestation stalls are not “welfare friendly,” and a future nationwide requirement may be that pregnant sows be housed in groups, as is soon to be required in some states. If the requirement for group housing were realized, the challenge for the swine industry would be maintenance of productivity of group-housed sows.
A disadvantage of group housing sows includes an inability to control individual sow feed intake. This would potentially lead to a greater variation in sow body condition which may, in turn, adversely affect sow fertility.2-4 Also, grouping of unfamiliar pregnant sows results in considerable aggression during the 2 to 3 days required for the establishment of social hierarchies.5,6 In addition to posing a welfare risk from injuries, the stress associated with the aggression may reduce sow fertility.7
There has been considerable earlier research on the impact of group housing of pregnant sows, and sow gestation housing has been the subject of a recent extensive review.8 It is not the intent to repeat here the latter review, other than to indicate that when well managed, including maintenance of stable sow groups, group housing of gestating sows can result in performance equal to8 or on occasion superior to9 that observed with individual housing. However, previous workers10-13 tended to employ few sows and small groups, usually with some sort of individual feeding system. The use of individual feeding systems will significantly add to the cost of a retrofit of existing operations. We are not aware of any controlled studies of sow performance comparing group-feeding of large sow groups to individual housing and feeding, under commercial conditions. Therefore, we undertook the present study to examine the hypothesis that housing sows in large groups from mid gestation under commercial conditions will not result in increased measures of aggression or stress and that pregnancy outcomes will remain unaffected.
Material and methods
Animals
These experiments were approved by the Michigan State All University Committee on Animal Use and Care and were conducted in a commercial farrow-to-wean facility housing 2500 sows of Yorkshire and Landrace breeding. Following a 17-day lactation, sows were weaned into individual gestation stalls and had 5 minutes of nose-to-nose contact with a boar each day, starting the day after weaning. Sows were artificially inseminated with commercial semen (containing 3 × 109 sperm) in the presence of a boar at first detection of estrus and then at 24-hour intervals if still exhibiting estrous behavior. Pregnancy was confirmed by transabdominal real-time ultrasound (RTU; Bantam; EI Medical, Loveland, Colorado) at 25 days after insemination.
Experiment One
Study design. Between days 65 and 70 of gestation, 96 sows were assigned to six experimental treatments in a 2 × 3 factorial design. Housing environment (group or stall) and parity (gilts, P0; primiparous sows, P1; and third parity sows, P3) were the main effects. The choice of parities was based on our unpublished observation that housing more gilts in a group increased aggression. Therefore, we hypothesized that younger animals fight more. We anticipated a relatively large difference between P0 and P1, with a lesser difference between P1 and P3. Four replicates of 24 sows were used (four animals per parity within treatment). Prior to relocation, all sows were individually identified using black hair dye, and their pregnancy status was re-confirmed by RTU.
Four large pens (7.8 m × 13.7 m) were used, that were approximately 70% slats and 30% solid concrete flooring. Each pen contained 32 drop-feeders, which supplied three feeder troughs across the width of each pen. The 12 sows in the group-housing environment were mixed with 38 other sows to form groups of 50, which allowed approximately 2.1 m2 per sow. Data were not collected for the 38 non-study sows. The average parities of all study and non-study sows in replicates 1, 2, 3, and 4 were 3.0, 2.3, 2.9, and 2.9, respectively. The stall-housed animals were relocated on the same day as sows were grouped, in such order that all animals were housed adjacent to an unfamiliar animal within the same parity and treatment in groups of four (ie, buffer sow, four treatment sows, buffer sow, four treatment sows). Food was provided twice daily (at 5:30 am and 11:30 am) for sows in both group and individual housing. Group-housed animals were provided water ad libitum by six nipple drinkers per pen, while stall-housed sows were given water approximately three times per day in the feed trough.
Behavioral observation. All experimental animals were observed continuously on each of 2 consecutive days following relocation, in three 1.5-hour time blocks starting at 6:00 am, 9:00 am, and 12:00 noon. The first observation period began immediately after the sow groups were formed. All occurrences of fights (defined as the reciprocal occurrence of head knocks, bites, or both) and attacks (defined as the one-sided occurrence of head knocks, bites, or both) involving the experimental animals within these time blocks were recorded by two observers. On the third day after relocation, feeding-time aggression for stalled and group-housed sows (quantified as above) was observed during each of two 30-minute feeding periods, starting at the time feed was dropped. Prior to analysis, the total number of active aggressive encounters (attacks and fights; ACT), passive aggressive encounters (received attacks; PAS), and the total number of aggressive encounters (active and passive encounters; AGG) were calculated as the number per hour.
Lesion scoring. One day before relocation and on the day after relocation, all experimental animals were scored for lesions by counting the total number of lesions on the head, neck, shoulders, and body. Lesion scoring was weighted, ie, minor skin abrasions were scored as 1, small punctures as 2, and bigger open lesions as 3. The difference between pre-relocation and post-relocation lesion scores was calculated prior to analysis.
Saliva sampling and cortisol analysis. Saliva samples
were obtained from unrestrained study sows 1 day before mixing and
on the day after relocation at 5:00 am,
8:00 am, 11:00 am, and 2:00 pm by inserting a piece of gauze
attached to a rubber tube into the mouth of the sow until
thoroughly moistened. Due to time constraints, only three of every
four animals were sampled, the sampled animals being selected
haphazardly. All samples were obtained within 30 minutes. The gauze
was then stored in a polypropylene centrifuge tube and kept at -4°C
until centrifugation at the end of the day. The samples were thawed
in a refrigerator and centrifuged at 1000g for 5 minutes.
The saliva was then aliquotted into 1.5-mL Eppendorff tubes and
stored at -20°C until analyzed. Salivary cortisol concentrations
were determined by radioimmunoassay (Coat-a-Count; Diagnostic
Products Corp, Los Angeles, California) modified for use in
pigs.14 Assay sensitivity was 0.28 nmol per L, and
intra-assay and interassay coefficients of variation were 5.9% and
10.8%, respectively. For the purposes of this study, hormone
amplitude was defined as the difference between the highest and
lowest cortisol concentrations within each day.
Experiment Two
Study design. A total of 937 mixed-parity sows in 10 weekly breeding groups between August and December were chosen randomly to be housed in groups of approximately 50 (n = 462) or housed individually in gestation stalls (n = 475). Sow management was as described for Experiment One. For 140 individually housed and 330 group-housed sows (“test sows”), backfat depths at the P2 position (65 mm off the midline at the last rib) were determined at 55 to 60 days of gestation, at farrowing, and at weaning using A-mode ultrasonography (Leanmeater; Renco, Minneapolis, Minnesota). The total and liveborn litter sizes were recorded for each of the 470 test sows. Additionally, the farm database was accessed to allow determination of farrowing rates for all 937 sows in the 10 breeding groups.
Statistical analysis
All analyses were performed using SAS version 8.2, 2001 (SAS Institute Inc, Cary, North Carolina). Results are expressed as means ± SEM, and P < .05 was considered significant. For Experiment One, the differences between pre-relocation and post-relocation salivary cortisol levels were determined for each pair of samples, and effects of housing environment (group or stall), parity (P0, P1, or P3), and their interactions were analyzed using a general linear mixed model, allowing for random effects of parity group, animal, and replicate. For analysis, each series of four stalls was considered a parity group for the stall-housed animals. Due to the buffer animals, this allowed effects of aggression to be limited to within-parity effects.
Effects of housing environment, parity, and their interactions on lesion scores were analyzed using ANOVA t-tests. Effects of housing environment, parity, and their interactions on behavior were analyzed using a general linear model, allowing for random effects of pen and replicate. For the stall-housed sows, each group of 12 animals was considered to be one housing group. This was so defined in order to be able to statistically account for differences caused by nontreatment pen mates (in group housing) or buffer animals (in stall housing). Correlations between lesion scores, cortisol concentrations, and aggressive encounters were analyzed using Spearman’s correlation test. Differences between feeding-time aggression and aggression on the day after relocation were analyzed using a paired t-test.
For Experiment Two, effects of housing environment on farrowing rate were compared using chi-square tests. Data for treatment effects on sow backfat depths, litter sizes, and wean-to-estrus intervals were examined by split-plot ANOVA, where breeding group served as the experimental unit for treatments but as a blocking factor for parities. Total born litter size was included as a co-variable in the analysis of liveborn litter size.
Results
Experiment One
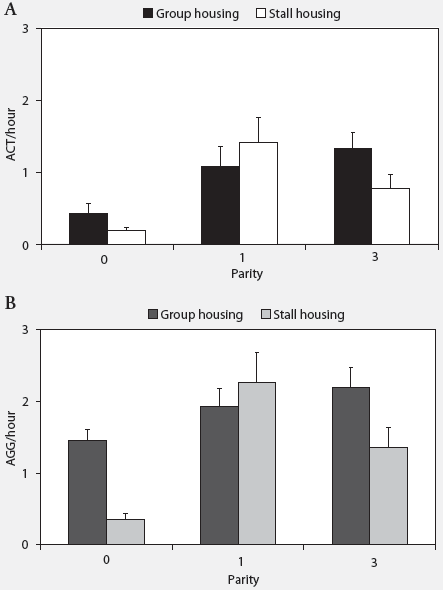
Behavior. There was no effect of housing environment on the number of post-relocation ACT. Group-housed animals (1.90 ± 0.27 ACT) did not fight with other animals more often than stall-housed animals (1.59 ± 0.29 ACT; P = .30). Parity did affect ACT, both for group-housed (P < .01) and stall-housed sows (P < .001). The P0 sows fought significantly less than the P1 (P < .001) and P3 (P < .001) sows both in groups and in stalls (Figure 1).
The AGG were affected by housing environment, with group-housed sows (3.71 ± 0.29 AGG) being involved in more aggressive encounters than stall-housed animals (2.65 ± 0.41 AGG; P < .05). Parity affected AGG differently in group-housed and stall-housed sows (housing environment × parity; P < .01). Whereas in group-housed sows, no significant differences between sows of different parities could be found, in stalls, P0 animals were involved in fewer aggressive encounters than P1 (P < .001) and P3 animals (P < .01) (Figure 1). The AGG per hour in group-housed sows were higher during feeding time (6.67 ± 0.70) than during the day of relocation (2.58 ± 0.22; P < .001), whereas in stall-housed animals, no significant differences in AGG per hour were found (feeding time, 1.69 ± 0.38; day of relocation, 1.54 ± 0.26; P = .26).
| Figure 1: Effects of housing environment and parity
on the mean number (± SEM) of (A) active aggressive encounters
(ACT) and (B) total aggressive encounters (AGG) after relocation of gilts
(P0) and sows of parity one (P1) and three (P3) (16 sows per parity per
housing treatment). Effects of housing environment (group housing or stall
housing), parity, and their interactions were analyzed using a general
linear mixed model. For both group and stall housing, number of ACT was
lower for P0 than for P1 and P3 sows (P < .001). Number of AGG
in group-housed sows was not affected by parity, but in stall-housed sows
was lower in P0 than in either P1 (P < .001) or P3 (P <
.01)
|
Lesions. The post-relocation increase in lesion score was higher for group-housed sows (22.52 ± 2.31) than for stall-housed animals (1.98 ± 0.40; P < .001), while the effect of parity on the increase in lesion scores tended towards significance (P = .08). In group-housed animals, the total increase in lesion score was positively correlated with AGG (P < .001) and with ACT (P < .01), whereas in stall-housed animals, the total increase was not correlated with either AGG (P = .24) or ACT (P = .32). Passive aggressive encounters were not correlated with the total increase in lesion score in either a group-housing (P = .23) or stall-housing environment (P = .32).
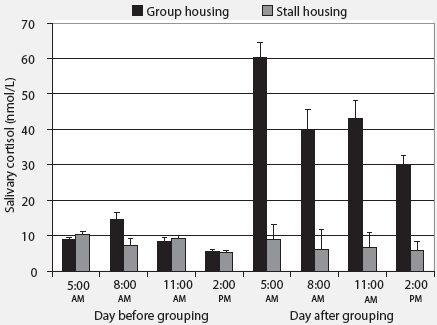
Cortisol concentrations. Housing environment affected the average increase in cortisol concentration after relocation (P < .01; Figure 2). Animals moved into a group-housing system showed a marked increase in salivary cortisol concentrations (33.4 ± 3.3 nmol per L), while animals relocated to a novel stall showed a slight decrease in salivary cortisol concentration (-1.38 ± 0.55 nmol per L). Parity did not affect the change in cortisol concentration after relocation (P = .55). Salivary cortisol concentration changed during the day, and the amplitude of this change differed between group-housed and stall-housed sows (P < .001). The difference between the highest and lowest cortisol concentration was greater for group-housed animals (59.6 ± 8.8 nmol per L) than for stall-housed animals (6.1 ± 0.55 nmol per L). Parity did not affect the difference (P = .32).
| Figure 2: Effect of housing environment on salivary
cortisol concentrations (nmol/L ± SEM) in group-housed (n
= 36) and stall-housed sows (n = 36) on the day of grouping and the following
day. Differences between pre-relocation and post-relocation salivary cortisol
levels were determined for each pair of samples, and effects of housing
environment, parity, and their interactions were analyzed using a general
linear mixed model allowing for random effects of parity group, animal,
and replicate. Average increase in salivary cortisol after relocation was
greater in group-housed than in stall-housed sows (P < .01).
|
Differences between pre- and post-relocation cortisol concentrations were not correlated with ACT, PAS, or AGG, regardless of housing environment (group-housed sows: ACT, P = .90, PAS, P = .25, and AGG, P = .51; stall-housed sows: ACT, P = .85, PAS, P = .54 and AGG, P = .79), nor was it correlated with lesion score (group-housed sows, P = .85; stall-housed sows, P = .31).
Experiment Two
There was no evident effect of housing management on P2 backfat depths (18.9 mm versus 17.7 mm, 18.6 mm versus 19.3 mm, and 16.4 mm versus 16.3 mm for stall- and group-housed sows at 55 to 60 days of gestation, farrowing, and weaning, respectively). There was no effect of housing management on farrowing rate (77.8% versus 76.6% for stall-housed and group-housed sows, respectively) or on subsequent litter size, although liveborn litter sizes tended to be larger in stall-housed sows (10.5 ± 0.8 versus 9.7 ± 0.2; P = .08). The subsequent wean-to-estrus interval was not affected by gestation housing management (10.2 ± 0.8 days versus 10.5 ± 0.5 days for stall-housed and group-housed sows, respectively).
Discussion
This study demonstrated that feeding time was an important factor affecting the higher levels of aggression recorded in group-housed animals than in sows housed in gestation stalls. This is in agreement with other recent data.15 Therefore, in order for the welfare of sows kept in groups to be maintained, better systems for delivery of food need to be developed. Floor feeding is an inexpensive alternative, but it favors the onset of aggressive interactions. Older sows initiated most of the aggressive interactions, both in individual and group housing. The opportunity for social encounters is significantly greater for sows housed in groups, and aggressive interactions are expected. Even considering that sows kept in stalls each have only two adjacent sows with which to interact, significant levels of aggression were recorded. The clear pattern of aggression at feeding time offers the possibility for strategic intervention by developing better feed delivery systems for group-housed sows.
The differences recorded in the daily salivary cortisol pattern of sows kept in groups and gestating stalls is intriguing and supports a previous report.16 Sows kept in gestation stalls had lower levels of salivary cortisol and showed smaller increases in cortisol levels in response to the relocation process than sows kept in groups. The cortisol data generated in the current study and in additional studies we have undertaken (data not shown) indicate no direct link between cortisol and sow fertility.
Within the limits of this study (ie, grouping for approximately the last 50 days of gestation), these data do not support the suggestion that group housing of sows during gestation will necessarily increase the absolute or variation in backfat depth. Therefore, solely on the basis of backfat depth as a measure of variation in sow feed intake, group housing pregnant sows appears to induce no adverse effects. Farrowing rate appeared unaffected by grouping sows within the last 50 days of gestation. It is expected that mixing sows in earlier gestation (eg, in dynamic groups) will reduce farrowing rate. The stage of gestation when sows can be mixed without detriment to pregnancy outcome has yet to receive attention, although on the basis of these data, 50 days does appear safe. The possibility of an adverse effect on liveborn litter size requires further investigation, since, if confirmed, it would be of economic significance. An etiology for this potential effect is not known. It has been suggested that sow fertility may suffer if stress is sufficiently prolonged,17 such as appears to be evident in the present study, although others noted no adverse effect on fertility of repeatedly mixing groups of pregnant gilts at 7-day intervals.18 On the basis of the data from the present study, we suggest that rehousing mid-gestation sows in large groups does not adversely impact fertility. However, the earliest stage of gestation at which mixing will not impact fertility remains to be determined.
Implications
- If sows are to be grouped during gestation, particular attention should be directed toward feeding management to avoid excessive aggression and possible adverse effects on welfare.
- Mixing sows into large groups at 50 days of gestation does not adversely affect fertility.
- The earliest stage of gestation at which mixing will not affect
fertility remains to be determined.
Acknowledgements
We are indebted to Dr Tina Widowski for her comments and advise regarding data presentation. We gratefully acknowledge the National Pork Board for financial support of this study.
References
1. English PR, Edwards SA. Animal welfare. In: Straw BE, D’Allaire S, Mengeling WL, Taylor DJ, eds. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:1067–1076.
2. Aherne FX, Kirkwood RN. Nutrition and sow prolificacy. J Reprod Fertil. 1985;33(suppl):169–183.
3. Kirkwood RN, Baidoo SK, Aherne FX. The influence of feeding level during lactation and gestation on the reproductive performance of second parity sows. Can J Anim Sci. 1990;70:1119–1126.
4. Young MG, Tokach MD, Aherne FX, Main RG, Dritz SS, Goodband RD, Nelssen JL. Comparison of three methods of feeding sows in gestation and the subsequent effects on lactation performance. J Anim Sci. 2004;82:3058–3070.
5. Mendl M, Zanella AJ, Broom DM. Physiological and reproductive correlates of behavioural strategies. Anim Behav. 1992;44:1107–1121.
6. Arey DS, Edwards SA. Factors influencing aggression between sows after mixing and the consequences for welfare and production. Livest Prod Sci. 1998;56:61–70.
7. Kongsted AG. Stress and fear as possible mediators of reproduction problems in group housed sows: a review. Acta Agric Scand A Anim Sci. 2004;54:58–66.
*8. McGlone JJ, von Borell EH, Deen J, Johnson K, Levis DG, Meunier-Salaun N, Morrow J, Reeves D, Salak-Johson JL, Sundberg PL. Compilation of the scientific literature comparing housing systems for gestating sows and gilts using measures of physiology, behavior, performance, and health. Prof Anim Sci. 2004;20:105–117.
9. Morris JR, Hurnik JF, Friendship RM, Evans NM. The effect of the Hurnik-Morris (HM) system on sow reproduction, attrition, and longevity. J Anim Sci. 1998;76:2759–2762.
10. Edwards SA. Scientific perspectives on loose housing systems for dry sows. Pig Vet J. 1992;28:40–51.
11. den Hartog LA, Backus GBC, Vermeer HM. Evaluation of housing systems for sows. J Anim Sci. 1993;71:1339–1344.
12. Broom DM, Mendl MT, Zanella AJ. A comparison of the welfare of sows in different housing conditions. Anim Sci. 1995;61:369–385.
13. Bates RO, Edwards DB, Korthals RL. Sow performance when housed either in groups with electronic sow feeders or stalls. Livest Prod Sci. 2003;79:29–35.
14. Ruis MA, Te Brake JH, Engel B, Ekkel ED, Buist WG, Blokhuis HJ, Koolhaas JM. The circadian rhythm of salivary cortisol in growing pigs: effect of age, gender, and stress. Physiol Behav. 1997;62:623–630.
15. Seguin MJ, Friendship RM, Kirkwood RN, Zanella AJ, Widowski TM. Effects of boar presence on agonistic behavior, shoulder scratches, and stress response of bred sows at mixing. J Anim Sci. 2006;84:1227–1237.
16. Zanella AJ, Broom DM, Hunter J, Mendl M. Brain opioid receptors in relation to stereotypies, inactivity and housing in sows. Physiol Behav. 1996;59:769–775.
17. Turner AI, Hemsworth PH, Tilbrook AJ. Susceptibility of reproduction in female pigs to impairment by stress and the role of the hypothalamo-pituitary-adrenal axis. Reprod Fertil Dev. 2002;14:377–391.
18. Soede NM, van Sleuwen MJW, Molenaar R, Rietveld FW, Schouten WPG, Hazeleger W, Kemp B. Influence of repeated regrouping on reproduction in gilts. Anim Reprod Sci. 2006;96:133-145.
*Non-refereed reference.