Original research |
Peer reviewed |
Acute and prolonged effects of ammonia on hematological variables, stress responses, performance, and behavior of nursery pigs
Efectos prolongados y agudos del amonio en variables hematológicas, respuestas al estrés, desempeño, y conducta en cerdos en destete
Effets aigus et prolongés de l’ammoniaque sur des variables hématologiques, les réponses au stress, les performances, et le comportement des porcelets en pouponnières
E. von Borell, PhD; A. Özpinar, PhD; K. M. Eslinger; A. L. Schnitz; Y. Zhao, PhD; F. M. Mitloehner, PhD
EVB: Institute of Agricultural and Nutritional Sciences, Martin-Luther-University Halle-Wittenberg, Halle, Germany. AO: Western Institute for Food Safety and Security, University of California, Davis, California. KME, YZ, FMM: Department of Animal Science, University of California, Davis, California. Corresponding author: Dr F. M. Mitloehner, One Shields Avenue, Davis, CA 95616–8521; Tel: 530-752-3936; Fax 530-752-0175; E-mail: fmmitloehner@ucdavis.edu.
Cite as: von Borell E, Özpinar A, Eslinger KM, et al. Acute and prolonged effects of ammonia on hematological variables, stress responses, performance, and behavior of nursery pigs. J Swine Health Prod. 2007;15(3):137–145.
Also available as a PDF.
SummaryObjectives: To determine acute and prolonged effects of 35 and 50 ppm concentrations of atmospheric ammonia (NH3) on welfare of weaned pigs. Materials and methods: Two experiments were conducted using gas exposure chambers to investigate prolonged effects (Experiment One; 19 days) and acute effects (Experiment Two; 96 hours) of NH3. Each experiment included two studies: exposure to NH3 at 0 and 35 ppm and at 0 and 50 ppm. In Experiment One, body weight, hematological and metabolic variables, and serum cortisol and haptoglobin were assessed, and behaviors were video-taped. In Experiment Two, serum cortisol and haptoglobin and plasma tumor necrosis factor-α were measured. Results: Absolute counts of white blood cells, lymphocytes, and monocytes were greater in pigs exposed to 35 ppm NH3 than in controls (P < .05). Serum haptoglobin was higher in pigs exposed to 50 ppm NH3 for 7 and 19 days than in controls (P < .05). Serum cortisol concentrations were greater in pigs exposed to 35 or 50 ppm NH3 for 19 days than in controls (P < .05). Less feeding behavior was observed in pigs exposed to 50 ppm NH3 than in controls (P < .05). In acute studies, serum cortisol concentrations were greater in pigs exposed to NH3 than in controls (P < .05). Implications: Under the conditions of these studies, prolonged exposure to NH3 is associated with increases in absolute monocyte, lymphocyte, and neutrophil counts and in serum cortisol and haptoglobin concentrations, but has no effect on pig growth performance. | ResumenObjetivos: Determinar los efectos prolongados y agudos de concentraciones de amonio atmosférico (NH3) en concentraciones de 35 y 50 ppm en el bienestar de cerdos destetados. Materiales y métodos: Se realizaron dos experimentos utilizando cámaras de exposición a gas para investigar los efectos prolongados (Experimento Uno; 19 días) y los efectos agudos (Experimento Dos; 96 horas) del NH3. Cada experimento incluyó dos estudios: exposición al NH3 a 0 y 35 ppm y a 0 y 50 ppm. En el Experimento Uno, se valoraron el peso corporal, las variables metabólicas y hematológicas, y el cortisol y la haptoglobina en el suero, y se videograbaron los comportamientos. En el Experimento Dos, se midieron el suero de cortisol, la haptoglobina, y el factor-α del plasma de la necrosis del tumor. Resultados: Los conteos absolutos de las células blancas de la sangre, los linfocitos, y los monocitos fueron mayores en los cerdos expuestos a 35 ppm de NH3 que en los controles (P < .05). La haptoglobina del suero fue mayor en cerdos expuestos a 50 ppm de NH3 por 7 y 19 días que en los controles (P < .05). Las concentraciones de cortisol en suero fueron mayores en cerdos expuestos a 35 o 50 ppm de NH3 por 19 días que en los controles (P < .05). Se observó un comportamiento de menor consumo de alimento en cerdos expuestos a 50 ppm de NH3 que en los controles (P < .05). En estudios agudos, las concentraciones de cortisol en suero fueron mayores en cerdos expuestos de manera aguda al NH3 que en los controles (P < .05). Implicaciones: Bajo las condiciones de estos estudios, la exposición prolongada al NH3 se relaciona con incrementos en los conteos absolutos de monocitos, linfocitos, y neutrófilos y en las concentraciones de cortisol y haptoglobina en suero, pero no tiene efecto en el desempeño de crecimiento el cerdo. | ResuméObjectifs: Déterminer les effets aigus et prolongés de concentrations de 35 et 50 ppm d’ammoniaque atmosphérique (NH3) sur le bien-être de porcs sevrés. Matériels et méthodes: Deux expériences ont été effectuées en utilisant une chambre permettant l’exposition au gaz pour étudier les effets prolongés (Expérience 1; 19 jours) et les effets aigus (Expérience 2; 96 heures) du NH3. Chaque expérience comportait deux études: exposition au NH3 à des concentrations de 0 et 35 ppm, et exposition à des concentrations de 0 et 50 ppm. Au cours de l’Expérience 1, on a mesuré le poids corporel, des variables hématologiques et métaboliques, et les concentrations sériques de cortisol et d’haptoglobine, et enregistré les comportements sur bande vidéo. Lors de l’Expérience 2, on a mesuré le cortisol et l’haptoglobine sériques et le facteur-α nécrosant de tumeurs. Résultats: Les nombres absolus de leucocytes, lymphocytes, et monocytes étaient plus élevés chez les porcs exposés à 35 ppm de NH3 que chez les porcs témoins (P < .05). L’haptoglobine sérique était plus élevée chez les porcs exposés à 50 ppm de NH3 pour 7 et 19 jours que chez les témoins (P < .05). Les concentrations de cortisol sérique étaient plus élevées chez les porcs exposés à 35 ou 50 ppm de NH3 pendant 19 jours que chez les témoins (P < .05). Moins de comportements de prise de nourriture ont été observés chez les porcs exposés à 50 ppm de NH3 que chez les témoins. (P < .05). Dans les études d’exposition aiguë, les concentrations de cortisol sérique étaient plus élevées chez les porcs exposés au NH3 que chez les témoins (P < .05). Implications: Dans les conditions expérimentales de ces études, une exposition prolongée au NH3 est associée avec une augmentation absolue des comptes de monocyte, lymphocyte, et neutrophile et des concentrations sériques de cortisol et d’haptoglobine, mais aucun effet n’a été observé sur les performances zootechniques des porcs. |
Keywords: swine, ammonia,
welfare, performance, stress response
Search the AASV web site
for pages with similar keywords.
Received: December
5, 2005
Accepted: May
31, 2006
Despite the lack in understanding of acute and prolonged effects of ammonia (NH3), it has been suggested that NH3 exposure increases inflammatory, immune, and neuroendocrine stress responses in pigs.1 The most common inflammatory pathway involves induction of cytokines (eg, IL-1, IL-4, IL-6, and tumor necrosis factor-α (TNF-α), which mediate and regulate immunity, inflammation, and hematopoiesis in response to tissue damage.2 Cytokines are produced de novo in response to an immune stimulus. Atmospheric NH3 is believed to cause release of cytokines by alveolar macrophages and neutrophils, constituting a potent inflammatory response.3 The early phase of inflammation is characterized by acute phase protein responses. An increase in concentrations of acute phase proteins (eg, haptoglobin) generally occurs during infection, injury, and tissue destruction; thus, acute phase proteins are useful stress indicators.4 High concentrations of haptoglobin and cytokines, and elevated counts of total white blood cells (WBC), macrophages, neutrophils, and lymphocytes, are generally viewed as indicators of inflammatory or immunological responses to stress.1 Serum cortisol, as a measure of the hypothalamic-pituitary-adrenal axis, is widely used to describe the effect of a stressor on immune function.5 Previous research has suggested that inflammatory stress correlates with suboptimal feed intake and growth.6
Current recommendations established by the US Occupational Safety and Health Administration (OSHA) on upper limits for NH3 concentrations in swine confinement buildings are mainly intended to provide occupational exposure limits over an 8-hour period. The OSHA threshold for permissible worker 8-hour exposure is 50 ppm, and the short-term exposure limit (15 minutes) is 35 ppm.7 While these two exposure standards relate to human health exposure, information on the effects of NH3 on animal welfare is scarce, and no thresholds have been established in the United States to date.8
The objectives of this study were to determine the effects of acute and prolonged exposure to atmospheric NH3 at concentrations of 35 and 50 ppm on welfare of recently weaned nursery pigs housed under controlled experimental conditions in environmental chambers. Welfare measurements included stress indices, hematological, metabolic, and endocrine variables, and growth performance and behavior.
Materials and methods
Animals, housing, and feeding
For each of four studies, male and female crossbred piglets (Yorkshire × Hampshire) weaned at 19.2 ± 1.1 days of age were distributed evenly by litter and gender into six pens (1.2 m × 1.2 m; four pigs per pen) in each of two chambers (24 pigs per study per chamber, 12 males and 12 females per chamber). Pigs were adapted to the housing conditions for 10 days after weaning, and exposure studies began when they were on average 29 days old.
The research was conducted at the Swine Research Teaching and Outreach Facility at the University of California, Davis, utilizing two identical environmental exposure chambers, each measuring 10.7 m long × 4.8 m wide × 3.1 m high (159 m3). One chamber (treatment chamber) was supplied with NH3 at concentrations of 35 and 50 ppm, and the other (control chamber) was supplied with fresh air (0 ppm NH3). Each chamber ceiling had two inlet air ducts and one outlet air duct. Fresh outside air (37.4 m3 per minute) was supplied through the inlet air ducts to each chamber and the same quantity of chamber air exited from the outlet air duct. Incoming air was unaltered except for heating or cooling. Room temperatures in each chamber were automatically maintained at 22°C ± 2°C. The slatted chamber floor was hosed clean with water once daily to remove excreta.
Each pen was equipped with a nipple waterer and a two-hole feeder that allowed access for two to three pigs to feed at any given time. In order to maintain ad libitum feed access, feeder reservoirs were re-filled once daily with a pelleted, corn-soy-based diet with 19% crude protein (as fed). Feed ingredients (on a dry matter basis) were corn (58.3%), soybean (26.5%), Akey Start 200 Base (Akey, Lewisburg, Ohio) (8%), fat (5%), mono-dical phosphate (1.2%), limestone meal (0.9%), salt (0.9%), and Tylan 40 (Elanco, Indianapolis, Indiana) (0.1%).
The University of California, Davis, Animal Care and Use Committee approved these studies.
Study design
Two experiments were conducted as completely randomized designs with pen as the experimental unit.9,10 Each experiment included 48 pigs, with 24 pigs and six replications per chamber, and four pigs per pen. Experimental design followed common pathology and exposure studies in which the impact of two housing environments for swine differing in pathological loads were compared.11,12
In Experiment One, two 20-day studies were conducted, beginning 10 days post weaning (Day 0). Prolonged effects on welfare were evaluated in groups of pigs exposed to atmospheric NH3 at 0 and 35 ppm in Study 1 and 0 and 50 ppm in Study 2. Blood samples and individual body weights (BW) were obtained Day -1 (pre ammonia exposure) and Days 7 and 19. Blood samples were obtained from all 24 pigs per chamber between 8:00 am and 9:00 am for cortisol, haptoglobin, and TNF-α assays. One pig per pen was randomly selected to be tested for hematology measures (n = 6). Behavior was video-taped between 7 am and 7 pm on Days 2 and 18, when blood samples were not collected, to ensure undisturbed behavior.
In Experiment Two, two 96-hour studies were conducted. Acute effects on welfare were evaluated in groups of pigs exposed to atmospheric NH3 at 0 and 35 ppm in Study 3 and at 0 and 50 ppm in Study 4. Blood samples were collected from all 24 pigs per treatment group at 72 hours before and 2, 8, 12, 24, 48, and 96 hours after ammonia exposure began, and six samples (one pig per pen, as in Experiment One) were randomly selected for testing for cortisol, haptoglobin, and TNF-α assays (n = 6)
Ammonia gas exposure
Before the experiments started, capability of the chambers to produce uniform gas distribution was assessed. Therefore, gas mixing characteristics were determined and found satisfactory using sulfur hexafluoride (SF6) tracer gas, which was released through the air inlet and measured in vertical and horizontal matrix planes. Chambers had forced ventilation at 3.8 × 104 L per minutes, resulting in a chamber residence time of approximately 6 minutes. The elevated NH3 concentration in the treatment chamber was achieved by mixing pure anhydrous NH3 gas (99.9% ammonia purity) with the fresh inlet air. The NH3 gas cylinder was located outside the treatment chamber and connected to the incoming air duct using Teflon tubing. A regulator controlled the delivery pressure, and a mass flow controller was used to adjust and monitor the NH3 flow rate. Swagelok fittings (Swagelok Company, Solon, Ohio) were used for all connections to prevent potential leaks of NH3 in the gas delivery system. The pure NH3 gas exited from the delivery tubing inside the inlet duct, where NH3 mixed with fresh air.
To achieve 35 and 50 ppm NH3 concentrations inside the treatment chamber, pure NH3 gas flow rates were 0.7 and 1.0 L per minute, respectively. Concentration of NH3 was monitored inside the animal pens at animal level using three instruments and methodologies. The first instrument, a Draeger Pac III NH3 gas monitor (Draeger, Pittsburgh, Pennsylvania; 1 ppm accuracy), was used three times per day. The second instrument, a Pranalytica photoacoustic spectroscopy monitor (Pranalytica, Santa Monica, California), measured NH3 concentrations continuously at animal level as described earlier by our laboratory.13 The third instrument was a Dionex ICS90 ion chromatograph (Dionex, Sunnyvale, California) using an acid impinger sampling method.13 For the latter method, air was sampled through Teflon tubing from the animal pens into sampling trains containing sulfuric acid. Atmospheric NH3 was trapped in the acid and analyzed in the laboratory using ion chromatography. The acid impinger method was conducted to confirm the Draeger Pac III and Pranalytica sensor measurements (Experiment One, twice per week; Experiment Two, 10 hours after exposure began). The NH3 flow rate was fine-tuned to keep the changes in NH3 concentration within 5% of the required values.
Blood-sample collection and processing
Blood samples for both experiments were collected via puncture of the anterior vena cava using evacuated blood collection tubes and 20-gauge, 3.8-cm disposable needles. Pigs were individually removed from the chamber and restrained on a bleeding table, and blood was collected in < 1 minute per pig, minimizing the stress associated with the procedure.
Whole blood samples from tubes containing sodium fluoride (for determination of lactate and glucose) were mixed by inversion and centrifuged (2500g for 5 minutes) within 15 minutes of collection. The separated plasma samples were immediately transported (on dry ice) to the laboratory for same-day analysis.
Whole blood samples from tubes that contained EDTA were kept cold on ice at 4°C and separated into two subsamples per pig. The first subsample was transferred to the laboratory on ice at 4°C for hematological measurements. The second subsample was centrifuged (2500g for 5 minutes) within 30 minutes of collection, and plasma was subdivided into two portions. The first plasma portion was transported on dry ice directly to the laboratory for metabolite analysis (ie, blood urea nitrogen [BUN], plasma NH3). The second plasma portion was stored at -70°C for analysis of TNF-α.
Whole blood samples for cortisol and haptoglobin determination were placed on ice for 2 hours before centrifugation (2500g at 4°C for 20 minutes) and the serum was stored at -70°C until analysis.
Hematology assays were conducted on the day the samples were collected. All frozen samples were thawed and assayed 2 days after the animal experiments were completed.
Hematology, clinical chemistry, and plasma NH3 analyses
All metabolite and cell determination measures were conducted at the Preclinical Research Service, Idexx Laboratories (West Sacramento, California). Plasma BUN, glucose, lactate, and NH3 concentrations were measured using an enzymatic method on an auto analyzer (Roche, Hitachi 717; Diamond Diagnostics, Holliston, Massachusetts). An automated cell counter (Coulter Gen-S Bayer, ADVIA 120 hematology analyzer; Diamond Diagnostics) was used for platelet count, red blood cell count, hemoglobin, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), WBC count, and absolute counts of neutrophils, lymphocytes, monocytes, eosinophils, and basophils.
Assays for TNF-α, haptoglobin, and cortisol
Plasma levels of TNF-α were measured in duplicate using a single commercial kit (swine-specific biotinylated monoclonal antibody sandwich ELISA; Biosource International, Swine TNF-alpha; Camarillo, California) according to the supplier’s instructions as described.14 Serum haptoglobin concentration was measured using a single commercial kit (Phase range haptoglobin assay kit; Tridelta Development, Greystones, Ireland) as described by Petersen et al.15 Serum cortisol concentration was measured in duplicate using a radioimmunoassay technique as previously described by Daley et al.16 Interassay and intra-assay coefficients of variation for TNF-α, haptoglobin, and cortisol were < 8%.
Behavior (Experiment One)
Two identical video systems (one per chamber) were installed to allow for detailed analysis of behaviors. One HTC 65°C day-night color camera per chamber (CCD image sensor, 380 TV lines, 1 lux sensitivity; Inter-Pacific, Deerfield, Illinois) with wide angle lens (CE F1.4/1.6–3.4 mm; Inter-Pacific) was bracket-mounted to the ceiling to cover the entire six-pen area (2.44 m × 3.66 m). One time-lapse video recorder (Samsung SLV-960A; Kyungki Do, Korea) per chamber was used to continuously record behavior in 24-hour time-lapse mode (2.78 mm tape per second, 12:1 compression). The four pigs in each pen were marked with an animal crayon marker (stripes, shoulder belts, spots, no marking) to allow for identification of individual pigs. Behavior data were analyzed on a per-pen basis using 10-minute scan sampling intervals for body positions and 5-minute scan sampling intervals for feeding behavior.17 Measured behaviors were directly entered from the video recordings into a computer spreadsheet.18 The list of measured behaviors (ethogram) included three categories: upright posture, defined as the pig assuming or maintaining an upright position on extended legs while standing still or moving; recumbency, the default behavior; and feeding behavior, measured and defined as the pig’s head positioned in the feeder. Data were expressed for each behavior category as its percentage of total observation time. Such data are generally not normally distributed. Therefore, the arcsine transformation was applied to the square roots of percentage data to achieve normal distribution before further parametric statistical analysis.18
Performance (Experiment One)
Measures related to growth performance were BW (kg per pen) and ADG (kg per pen). Individual BW was measured using a portable electronic scale (accuracy ± 0.02 kg). Measurements of feed intake (as fed) were attempted by collecting feed refusals from the feeders and floor and subtracting them from feed provided to the pigs. As feed residuals partly fell through the slatted floor where they mixed with excreta, feed refusals were not measured and feed efficiency could not be calculated.
Statistical analyses
Behavior, BW, and blood analyses-related data were analyzed as a split-plot for repeated measures (for day in Experiment One or time in Experiment Two) using PROC MIXED in SAS (SAS Institute Inc, Cary, North Carolina). The model included treatment (tested with pen-within-chamber variance), effects of day in Experiment One or time in Experiment Two, and the interaction of treatment × day (or time) in the subplot. Average-daily-gain data were analyzed using PROC GLM. The model included treatment and pen-within-chamber as the error term.
Results
Experiment One: prolonged NH3 exposure studies
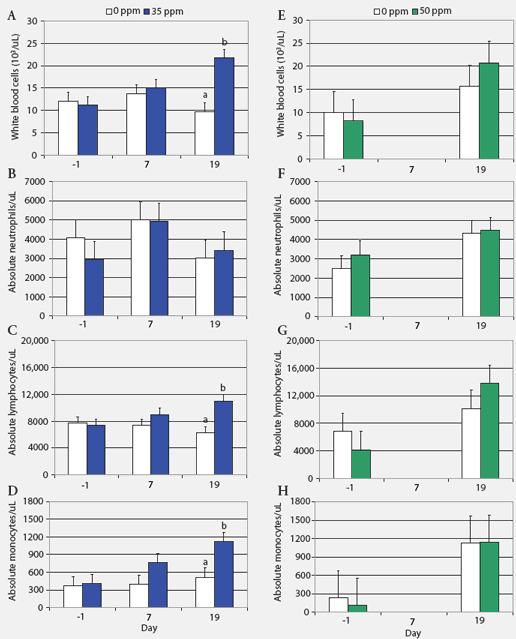
Hematology, biochemistry, and NH3 measurements. Absolute blood cell counts (Figure 1) and results for cortisol and haptoglobin assays (Figure 2) are expressed as least squares means. Prolonged exposure to atmospheric NH3 affected absolute blood cell counts (Figure 1). On Day 19, WBC and absolute numbers of lymphocytes and monocytes in pigs exposed to 35 ppm NH3 were approximately twice those in the control animals. Blood cell counts did not differ between groups exposed to 0 and 50 ppm NH3. Hemoglobin, MCV, MCH, and MCHC were similar for control groups and those exposed to NH3 (ranges 100 to 160 g per L, 50 to 68 fL, 17 to 23 pg, and 300 to 360 g per L, respectively). Concentrations of blood metabolites and plasma NH3 were similar in controls and pigs exposed to NH3 (BUN range, 3.3 to 3.6 mmol per L; glucose range, 4.9 to 5.3 mmol per L; lactate range, 5.29 to 6.84 mmol per L; NH3 range 25.7 to 47.0 μg per dL).
| Figure 1: White blood cell counts (least squares
means) in nursery pigs exposed to atmospheric ammonia for 19 days at 0
versus 35 ppm (panels A through D) and at 0 versus 50 ppm atmospheric ammonia
(panels E through H) (Experiment One; chronic exposure). Pigs weaned at
10 days of age were housed four per pen, with pen the experimental unit
(six pens and 24 pigs per treatment). Exposure to ammonia began 10 days
post weaning when the pigs were 29 days old (Day 0). Samples from one pig
per pen were tested on Days -1, 7, and 19 (n = 6). Values within a panel
with different letters differ (P < .05; ANOVA). Day 7 data for
pigs on 50 ppm ammonia are missing because whole blood samples were unintentionally
discarded.
|
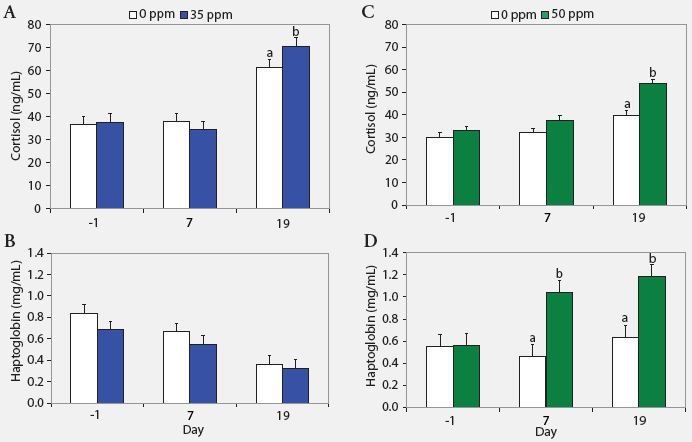
| Figure 2: Serum cortisol and haptoglobin concentrations
(least squares means) in nursery pigs (described in Figure 1) exposed to
atmospheric ammonia at 0 versus 35 ppm (panels A and B) and 0 versus 50
ppm (panels C and D) at Days -1, 7, and 19 (Experiment One; n = 24). Values
within a panel with different letters differ (P < .05; ANOVA).
|
Serum cortisol, haptoglobin, and TNF-α. Serum cortisol concentrations were greater on Day 19 (P < .05) in pigs exposed to either 35 or 50 ppm NH3 than in control animals (Figure 2). Additionally, haptoglobin was higher on Days 7 and 19 (P < .05) in pigs exposed to 50 ppm NH3 than in controls. Tumor necrosis factor-α was similar across treatments (range, 31 to 60 pg per mL).
Performance. Initial mean BW (Day 0) (± standard error of the mean [SEM]) were 9.95 ± 0.57 kg (Study 1) and 7.50 ± 0.32 kg (Study 2). On Day 19, BW of pigs exposed to 35 ppm NH3 (19.4 ± 0.95 kg) and control pigs (19.1 ± 0.95 kg) did not differ (P > .05; Study 1), and BW of pigs exposed to 50 ppm NH3 (12.4 ± 0.81 kg) and control pigs (12.9 ± 0.81 kg) did not differ (P > .05; Study 2). Accordingly, ADG did not differ between treatments in either study.
Behavior. Body posture, feeding, and aggressive behaviors on Day 2 were similar in pigs exposed to NH3 and control pigs. However, on Day 18, time spent feeding was less (mean + SEM) in pigs exposed to 50 ppm NH3 (11.27% ± 1.29% of time) than in control animals (12.27% ± 1.29% of time) (P < .05). Across both studies, the combined average percent feeding time for the control groups was 12.82% on Day 2 and 11.37% on Day 18. Finally, for all treatment groups, the range for upright posture (across both studies and on both observation days) was 30% to 42% of time, and the range for aggression was 0.4% to 2.2% of time.
Experiment Two: acute NH3 exposure studies
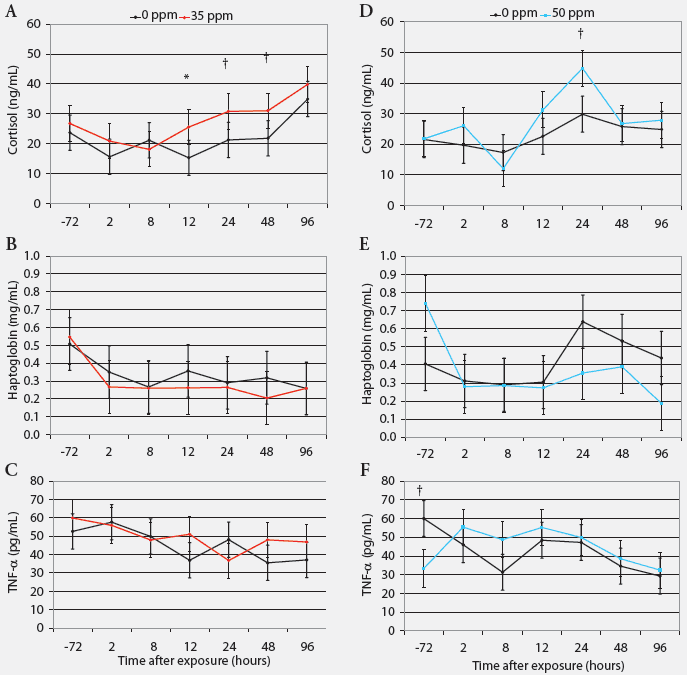
Cortisol, haptoglobin, and TNF-α. Serum cortisol was greater in pigs exposed to 35 ppm NH3 than in controls after 12 hours (P < .05), and tended to be greater after 24 hours (P = .07) and 48 hours (P = .08; Figure 3). Acute exposure to NH3 did not affect serum haptoglobin or plasma TNF-α concentrations (Figure 3).
| Figure 3: Serum cortisol, haptoglobin, and tumor
necrosis factor-α (TNF-α) in nursery pigs acutely exposed to
ammonia gas at 0 ppm versus 35 ppm and at 0 ppm versus 50 ppm for 96 hours
(least squares means; Experiment Two). Blood samples collected from six
pigs per treatment (one per pen) at 72 hours before and 2, 8, 12, 24, 48,
and 96 hours after ammonia exposure began were tested (n = 6). * Control
and treatment values differ (P < .05; ANOVA); †Control
and treatment values tend to differ (P < .10 ANOVA).
|
Discussion
The role of NH3 in development of respiratory disease remains unclear, although it acts synergistically with other pollutants and may influence the incidence and severity of pathogen-induced respiratory diseases.19 Ammonia is highly soluble in water and is presumably largely absorbed by the distal airway mucus. Ammonia can favor bacterial contamination of the lungs by decreasing pulmonary clearance and inducing airway mucosal inflammation.20-22 Ammonia also can affect cellular necrosis of alveolar tissues and lead to respiratory stress and edema. Stress in general has effects on immune, endocrine, behavior, and performance measures.23 Stress factors induce a series of natural defense reactions, which constitute homeostatic processes. The early phase of airway mucosal inflammation elicits an acute-phase response. Among the most prominent acute-phase responses is an increase in liver-synthesized serum proteins, ie, acute phase proteins, which are believed to play a vital role in the physiological stress response.4 Haptoglobin, an acute phase protein, plays a vital role in the restoration of homeostasis after injury, tissue necrosis, and infection by scavenging heme released by damaged cells. Increased serum concentrations of haptoglobin are also indicative of inflammatory or infectious lesions.24 Haptoglobin is generally regarded as being a sensitive, although non-specific, indicator of stress and is used to assess health in pigs.25 Grellner et al26 suggested that serum concentrations of acute phase protein in pigs are negatively correlated with BW, indicating that a prolonged activated cellular immune response is a detriment to growth. In our study, mean serum haptoglobin concentration of pigs exposed to 50 ppm of NH3 was twice that of their peers in the control chamber. This high haptoglobin concentration on Days 7 and 19 might indicate that the pigs exposed to 50 ppm of NH3 did not adapt to or recover from the gas stimulus, but invested significantly in the cleanup of cell debris. A continuing high haptoglobin concentration might indicate pulmonary edema or continuing alveolar necrosis; however, pigs exposed to 35 ppm did not show greater serum haptoglobin concentrations than the controls, which may indicate that these pigs detoxified after the initial insult. A combination of serum haptaglobin and serum cortisol concentrations may be a more reliable indicator of disease status or stress in pigs than either measurement alone.3 Interestingly, our experiments showed a tendency for pigs exposed to NH3 to have higher serum cortisol concentrations not only in the acute but also in the prolonged studies (Day 19). This is in contrast to the results of another study,27 which showed no cortisol response to exposure to concentrations of 25 to 100 ppm of atmospheric NH3 over a 6-day period.
Cytokines mediate a variety of local and systemic biological functions involved in the control of acute phase protein expression.2 Ammonia causes the release of cytokines by alveolar macrophages and neutrophils, constituting an inflammatory response.3 Correlations of haptoglobin and plasma TNF-α with prolonged stress were reported earlier.28 Our studies did not show a response of the cytokine TNF-α to prolonged or acute exposure to NH3, which may be explained by the large degree of variation in this parameter. In addition to cytokines and acute phase proteins, high total WBC count and absolute numbers of macrophages, neutrophils, and lymphocytes are considered indicators of immunological responses to respiratory stress.1 In the present studies, pigs exposed to NH3 at 35 ppm, compared to the controls, had much higher total WBC and absolute numbers of lymphocytes and monocytes, but numbers of neutrophils did not differ between treated and control groups. Absolute numbers of lymphocytes and monocytes were not consistently increased in groups exposed to 50 ppm in Study 2. We believe that the large degree of variance in these values masked what we expected to be significant differences (as exhibited in Study 1; exposure to 35 ppm).
The present experimental design followed those of common pathology and exposure studies11,12 in which multiple subjects are tested per exposure room or building and the animal or pen, rather than the room, is considered the experimental unit. In these studies, pen was the experimental unit, and in Experiment One, we randomly selected one pig per pen for hematology testing, considering this randomly selected animal as representative of the pen of four animals. In addition, we considered the process of removing individual pigs from the chamber and collecting blood samples from each pig while separated from the group to be less stressful than collecting samples from the pigs among their peers.
It should be noted that the treatment (ammonia concentration) was applied continuously in each ammonia treatment room in all four studies. In future studies, a larger sample size (ie, more pen replications) might be advantageous to increase statistical power, thereby addressing the issue of large variability, to determine whether similar effects occur in pigs exposed to NH3 at 50 and 35 ppm.
Previous research in nursery pigs has suggested that pro-inflammatory cytokines correlate with low feed intake and growth.6 Additionally, Drummond et al20 compared effects of 50, 100, and 150 ppm NH3 versus the control (0 ppm) on performance and reported ADG was lower by 12%, 30%, and 29%, respectively, compared to the controls. Our studies comparing pigs exposed to NH3 at 0 and 35 ppm and at 0 and 50 ppm did not detect effects on performance, other than a trend toward low dry matter intake at 50 ppm NH3 exposure. These results agree with those of others,8 who found no effects of chronic NH3 exposure (up to 37 ppm NH3) on productivity of weaned pigs > 5.5 weeks of age.
Animal behavior is regarded as a sensitive indicator of what an
animal prefers or
dislikes. Morrison et al29 concluded that NH3
concentrations in commercial buildings are not sufficient to induce
aversion to NH3. Although the experiment at hand does not address
ammonia aversion and preferences, more recent preference
tests30,31 indicate that weanling pigs did prefer to
avoid an area where NH3 was ≥ 20 ppm, but this avoidance
was delayed and explained by the possible development of a general
sense of malaise. Even operant responses of pigs to high
concentrations of NH3 (up to 100 ppm) revealed a relatively
weak aversion to polluted air exposure while they were rooting for
food.32 Although the concept of malaise in the context
of motivational studies was not the question of concern in our
work, one might expect that subclinically diseased pigs would
decrease their feeding behavior (frequency and duration of feeding
bouts) at NH3 concentrations that were previously reported to
affect the behavior of pigs. Our results, however, supported that
hypothesis only at 50 ppm and not at 35 ppm NH3 exposure. Pigs and
other species typically reduce their overall activity during
periods of inflammation, which is referred to as sickness
behavior.33 The lack of a difference in upright body
postures between pigs exposed to 35 or 50 ppm and untreated pigs in
Experiment One does not support the interpretation that pigs
exposed to these NH3 concentrations experienced a state of
sickness.
Current recommendations on upper NH3 limits are mainly intended to provide occupational exposure limits, as the scientific evidence that NH3 exposure affects animal health and performance is scarce.8,34 Synergistic effects of dust and NH3 on swine health35,36 and on occupational health of farm workers 35,37 have been reported and need to be considered accordingly. Recent studies failed to find an effect of a 5-week chronic exposure to NH3 (≤ 37 ppm) on respiratory disease in weaned pigs.36,37 Even after exposure to combinations of dust and NH3, gross pathology was minimal and widespread minor pathological changes were of little significance.36 Most existing guidelines and recommendations for animal houses set limits ranging from 20 to 50 ppm of NH3. Our studies indicate that pigs respond to NH3 with systemic stress responses; however, even 50 ppm does not affect animal growth performance over a 20-day period. Future studies should focus on the effects of NH3 on lung histopathology to determine the kind of damage occuring due to NH3 exposure that elicits the animal’s physiological stress response.
Implications
- Under the conditions of this study, prolonged exposure of weaned pigs to atmospheric NH3 elicits increases in WBC, absolute numbers of lymphocytes and monocytes, and serum cortisol and haptoglobin.
- Under the conditions of this study, exposure to NH3 at concentrations of up to 50 ppm does not affect weight gain of pigs.
- Reduced feeding behavior at exposure to 50 ppm NH3 implies that prolonged exposure (in combination with other factors) should be studied on a larger number of pigs.
Acknowledgements
This study was funded by the National Pork Board, Grant #03–159. The authors acknowledge the support for E. von Borell via a fellowship under the OECD Cooperative Research Program “Biological Resource Management for Sustainable Agriculture Systems.” The authors thank K. Parker, L. McDonnell, E. Veenendaal, and Leticia Valadez for their assistance.
References
1. Asmar S, Pickrell JA, Oehme FW. Pulmonary diseases caused by airborne contaminants in swine confinement buildings. Vet Hum Toxicol. 2001;43:48–53.
2. Kataranovski M, Magic Z, Pejnovic N. Early inflammatory cytokine and acute phase protein response under the stress of thermal injury in rats. Physiol Res. 1999;48:473–482.
3. Murata H, Horino R. Effects of in vitro atmospheric ammonia exposure on recovery rate and luminol-dependent chemiluminescence of bovine neutrophils and bronchoalveolar macrophages. J Vet Med Sci. 1999;61:279–281.
4. Weissman C. The metabolic response to stress: an overview and update. Anesthesiol. 1990;73:308–327.
5. Tuchscherer M, Puppe B, Tuchscherer A, Kanitz E. Effects of social status after mixing on immune, metabolic, and endocrine responses in pigs. Physiol Behav. 1998;64:353–360.
6. Spurlock ME. Regulation of metabolism and growth during immune challenge: An overview of cytokine function. J Anim Sci. 1997;75:1773–1783.
7. Alder RG. Ammonia in workplace atmospheres – solid sorbent. 2002. Available at: www.osha.gov/dts/sltc/methods/inorganic/id188/id188.html. Accessed 23 Jan 2007.
8. Wathes CM, Demmers TGM, Teer N, White RP, Taylor LL, Bland V, Jones P, Armstrong D, Gresham ACJ, Hartung J, Chennells DJ, Done SH. Production of weaned pigs after chronic exposure to airborne dust and ammonia. Anim Sci. 2004;78:87–97.
9. Cox DR. Planning of Experiments. New York: Wiley; 1958.
10. Aron DK, Hays VW. How many pigs? Statistical power considerations in swine nutritional experiments. J Anim Sci. 2004;82:245–254.
11. Williams NH, Stahly TS, Zimmerman DR. Effects of level of chronic immune system activation on the growth and dietary lysine needs of pigs fed from 6 to 112 kg. J Anim Sci. 1997;75:2463–2471.
12. Lee C, Giles LR, Bryden WL, Downing JL, Owens PC, Kirby AC, Wynn PC. Performance and endocrine responses of group housed weaner pigs exposed to the air quality of a commercial environment. Livest Prod Sci. 2005;93:255–262.
13. Webber ME, MacDonald T, Pushkarsky MB, Patel CK, Zhao Y, Marcillac N, Mitloehner FM. Agricultural ammonia sensor using diode lasers and photoacoustic spectroscopy. Meas Sci Technol. 2005;16:1–7.
14. Myers MJ, Farrell DE, Palmer DC, Post LO. Inflammatory mediator production in swine following endotoxin challenge with or without co-administration of dexamethasone. Int Immunopharmacol. 2003;3:571–579.
15. Petersen HH, Nielsen JP, Jensen AJ, Heegoard PM. Evaluation of an enzyme-linked immunosorbent assay for determination of porcine haptoglobin. J Vet Med A. 2001;48:513–523.
16. Daley CA, Sakuria H, Adams BM, Adams TE. Effect of stress-like concentrations of cortisol on gonadotroph function in orchidectomized sheep. Biol Reprod. 1999;60:158–163.
17. Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. 2nd ed. Cambridge, UK: Cambridge University Press; 1993.
18. Mitlohner FM, Morrow-Tesch JL, Wilson SC, Dailey JW, McGlone JJ. Behavioral sampling techniques for feedlot cattle. J Anim Sci. 2001;79:1189–1193.
19. Hamilton TDC, Roe JM, Hayes CM, Jones P, Pearson GR, Webster AFJ. Contributory and exacerbating roles of gaseous ammonia and organic dust in the etiology of atrophic rhinitis. Clin Diagn Lab Immunol. 1999;6:199–203.
20. Drummond JG, Curtis SE, Simon J, Norton HW. Effects of aerial ammonia on growth and health of young pigs. J Anim Sci. 1980;50:1085–1091.
21. Drummond JG, Curtis SE, Simon J, Norton HW. Effects of atmospheric ammonia on young pigs infected with Bordetella bronchiseptica. Am J Vet Res. 1981;42:963–968.
22. Drummond JG, Curtis SE, Meyer RC, Simon J, Norton HW. Effects of atmospheric ammonia on young pigs experimentally infected with Ascaris suum. Am J Vet Res. 1981;42:969–974.
23. Hicks TA, McGlone JJ, Whisnant CS, Kattesh HG, Norman RL. Behavioral, endocrine, immune and performance measures for pigs exposed to acute stress. J Anim Sci. 1998;76:475–483.
24. Lampreave F, Gonzalez-Ramon N, Martinez-Ayensa S, Hernandez MA, Lorenzo HK, Garcia-Gil A, Pineiro A. Characterization of the acute phase serum protein response in pigs. Electrophoresis. 1994;15:672–676.
25. Eckersall PD, Saini PK, McComb C. The acute phase response of acid soluble glycoprotein, alpha-acid glycoprotein, ceruloplasmin, haptoglobin, and C-reactive protein in the pig. Vet Immunol Immunopathol. 1996;51:377–385.
26. Grellner GF, Fangman TM, Caroll JA, Wiedmeyer CE. Using serology in combination with acute phase proteins and cortisol to determine stress and immune function of early-weaned pigs. J Swine Health Prod. 2002;10:199–204.
27. Gustin P, Urbain B, Prouvost JF, Ansay M. Effects of atmospheric ammonia on pulmonary hemodynamics and vascular permeability in pigs: Interaction with endotoxins. Toxicol Appl Pharmacol. 1994;125:17–26.
28. Harding JC, Baarsch MJ, Murtaugh MP. Association of tumor necrosis factor and acute phase reactant changes with post arrival disease in swine. J Vet Med B. 1997;44:405–413.
29. Morrison WD, Pirie PP, Perkins S, Braithwaite LA, Smith JH, Waterfall D, Doucett CM. Gases and respirable dust in confinement buildings and the responses of animals to such airborne contaminants. In: Collins E, Boon C, eds. Livestock Environment IV. Fourth International Symposium. St Joseph, Michigan: American Society of Agricultural Engineers; 1993:734–741.
30. Jones JB, Burgess LR, Webster AJF, Wathes CM. Behavioural responses of pigs to atmospheric ammonia in a chronic choice test. Anim Sci. 1996;63:437–445.
31. Wathes CM, Jones JB, Kristensen HH, Jones EKM, Webster AJF. Aversion of pigs and domestic fowl to atmospheric ammonia. Trans Am Soc Agric Eng. 2002;45:1605–1610.
32. Jones JB, Burgess LR, Webster AJF, Wathes CM. Operant responses of pigs to atmospheric ammonia. Appl Anim Behav Sci. 1998;58:35–47.
33. Johnson RW, von Borell E. Lipopolysaccharide-induced sickness behavior in pigs is inhibited by pretreatment with indomethacin. J Anim Sci. 1994;72:309–314.
34. Donham KJ. Association of environmental air contaminants with disease and productivity in swine. Am J Vet Res. 1991;52:1723–1730.
35. Donham KJ, Leininger JR. Animal studies of potential chronic lung disease of workers in swine confinement buildings. Am J Vet Res. 1984;45:926–931.
36. Done SH, Gresham ACJ, Williamson S, Hunt B, Chennells DJ, White RP, Demmers TGM, Teer N, Wathes CM, Taylor L, Bland V, Jones P, Armstrong D. The pathological findings in pigs exposed to aerial pollutants. Pig J. 2003;51:119–130.
37. Done SH, Chennells DJ, Gresham ACJ, Williamson S, Taylor LJ, Bland V, Hunt B, Jones P, Armstrong D, White RP, Demmers TGM, Teer N, Wathes CM. Clinical and pathological responses of weaned pigs to ammonia and dust. Vet Rec. 2005;157:70–80.