Original research |
Peer reviewed |
Effects on growth performance, feed efficiency, and health of weanling pigs fed fermented liquid whey inoculated with lactic acid bacteria that inhibit Escherichia coli in vitro
Efectos en el desempeño del crecimiento, eficiencia alimenticia, y salud de cerdos recién destetados alimentados con suero de leche líquido fermentado inoculado con bacteria de ácido láctico que inhibe in vitro a la Escherichia coli
Effets du lactosérum fermenté inoculé avec des bactéries lactiques inhibant Escherichia coli in vitro sur les performances de croissance, l’efficacité alimentaire, et la santé de porcelets sevrés
Maria del Rocio Amezcua, MVZ, EPA, MSc, PhD; Robert Friendship, DVM, MSc, Diplomate ABVP; Catherine Dewey, DVM, MSc, PhD; J. Scott Weese, DVM, DVSc, Diplomate ACVIM; Cornelius de Lange, MSc, PhD; Gregor Reid, MBA, PhD
MDRA, RF, CD: Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada. JSW: Department of Clinical Studies, Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada. CDL: Department of Animal and Poultry Science, University of Guelph, Guelph, Ontario, Canada. GR: Lawson Health Research Institute, University of Western Ontario, London, Ontario, Canada. Dr Reid owns patents on Lactobacillus strains GR-1 and RC-14, unrelated to pigs or animal care. Corresponding author: Dr Robert Friendship, Department of Population Medicine, Ontario Veterinary College, University of Guelph, Guelph, Ontario, Canada N1G 2W1.
Cite as: Amezcua MDR, Friendship R, Dewey C, et al. Effects on growth performance, feed efficiency, and health of weanling pigs fed fermented liquid whey inoculated with lactic acid bacteria that inhibit Escherichia coli in vitro. J Swine Health Prod. 2007;15(6):320–329.
Also available as a PDF.
SummaryObjectives: To determine the fermentation dynamics of liquid whey-dextrose (FLWD) inoculated with lactic acid bacteria (LAB) and whether feeding FLWD inoculated with LAB and added to a basal dry diet without antibiotics affects growth, feed efficiency, and health of weanling pigs. Materials and methods: One hundred and forty newly weaned pigs were assigned to five dietary treatments (four pens of seven pigs per treatment). Three FLWD preparations inoculated with either human- or pig-origin LAB strains were added to a basal dry feed. The fourth FLWD preparation contained no LAB. The fifth diet was the basal dry feed containing 0.1% lincomycin (control). LAB strains were mixed with FLWD prior to fermentation. Dry matter (DM), pH, and LAB counts of diets were measured daily during the 5-day fermentation period and the first 2 days of storage. Growth performance was recorded and rectal swabs were collected weekly. Fecal consistency was evaluated daily. Results: The pH and DM of fermented feed decreased and total LAB increased over time. Average daily gain and feed intake were highest in controls. Prevalence and severity of diarrhea were greater in pigs consuming LAB-inoculated diets than in control pigs. Mortality did not differ among treatment groups. Fewer hemolytic Escherichia coli were recovered from pigs fed FLWD. Implications: Fermented liquid feeds do not consistently promote better growth performance and health in weanling pigs. Use of LAB in starter feed may inhibit enteric E coli; however, further studies are needed to determine whether specific strains of LAB may prevent postweaning diarrhea. | ResumenObjetivos: Determinar la dinámica de fermentación de la dextrosa de suero líquido (FLWD por sus siglas en inglés) inoculada con bacterias de ácido láctico (LAB por sus siglas en inglés) y determinar si el alimentar con la FLWD inoculada con LAB agregada a una dieta base seca sin antibióticos afecta el crecimiento, la eficiencia alimenticia, y la salud de los cerdos recién destetados. Materiales y métodos: Ciento cuarenta cerdos recién destetados fueron asignados a cinco tratamientos dietéticos (cuatro corrales de siete cerdos por tratamiento). Se agregaron tres preparaciones de FLWD inoculadas con cepas de LAB de origen humano o de cerdo a un alimento base seco. La cuarta preparación de FLWD no contenía LAB. La quinta dieta fue el alimento base seco que contenía 0.1% de lincomicina (control). Se mezclaron las cepas de LAB con FLWD antes de la fermenatación. Diariamente se midieron la materia seca, el pH, y el conteo de LAB de cada dieta durante los 5 días del periodo de fermentación y los 2 primeros días de almacenaje. Se registró el desempeño del crecimiento de los cerdos y se recolectaron hisopos rectales semanalmente. La consistencia fecal se evaluó diariamente. Resultados: El pH y la DM (materia seca por sus siglas en inglés) del alimento fermentado disminuyó y la LAB total aumentó con el tiempo. El grupo control presentó la ganancia diaria promedio y el consumo de alimento más altos. La prevalencia y severidad de la diarrea fueron mayores en los cerdos que consumieron alimento inoculado con LAB. La mortalidad no difirió entre los tratamientos. Se recuperó menos Escherichia coli hemolítica de los cerdos alimentados con FLWD. Implicaciones: Los alimentos líquidos fermentados no promueven consistentemente un mejor desempeño del crecimiento y salud en cerdos recién destetados. El uso de LAB en alimento de iniciación puede inhibir la E coli entérica; sin embargo, se necesitan estudios adicionales para determinar si las cepas específicas de LAB pueden prevenir la diarrea post destete. | ResuméObjectifs: Déterminer les dynamiques de fermentation de lactosérum-dextrose liquide (FLWD) inoculé avec des bactéries lactiques (LAB) et déterminer si l’ajout de FLWD inoculé avec des LAB à la diète sèche de base sans antibiotique influençait la croissance, l’efficacité alimentaire, et la santé de porcelets sevrés. Matériels et méthodes: Cent quarante porcelets récemment sevrés ont été répartis dans cinq groupes de traitement alimentaire (quatre parcs de sept porcs par traitement). Trois préparations de FLWD inoculées avec des souches de LAB d’origine humaine ou porcine ont été ajoutées à la diète sèche de base. La quatrième préparation de FLWD ne contenait aucune LAB. La cinquième diète était constituée de la diète sèche de base additionnée de 0,1% de lincomycine (témoin). Les souches de LAB étaient mélangées avec le FLWD avant la fermentation. La quantité de matière sèche, le pH, et le dénombrement de LAB de chaque diète étaient mesurés quotidiennement durant la période de fermentation de 5 jours et les deux premiers jours d’entreposage. Les performances de croissance des porcs étaient enregistrées et des écouvillons rectaux prélevés à chaque semaine. La consistance fécale était évaluée quotidiennement. Résultats: Le pH et la quantité de matière sèche des aliments fermentés ont diminué et le nombre de LAB total a augmenté avec le temps. Le gain quotidien moyen et la quantité de nourriture ingérée étaient les plus élevés chez les témoins. La prévalence et la sévérité de la diarrhée étaient plus élevées chez les porcs consommant de la nourriture inoculée avec le LAB. Le taux de mortalité ne différait pas parmi les groupes d’animaux traités. Une quantité moindre d’Escherichia coli hémolytique a été isolée des porcs nourris avec FLWD. Implications: Les aliments liquides fermentés ne favorisent pas de manière constante de meilleures performances de croissance et la santé de porcelets sevrés. L’utilisation de LAB dans les aliments de départ peut inhiber les E coli entérique; toutefois, des études supplémentaires sont nécessaires afin de déterminer si des souches spécifiques de LAB peuvent prévenir la diarrhée en période post-sevrage. |
Keywords: swine, probiotics, Escherichia
coli, growth, fermented liquid whey
Search the AASV web site
for pages with similar keywords.
Received: December
6, 2006
Accepted: May
2, 2007
The potential removal of antimicrobials from farm-animal feeds has stimulated a renewed interest in the use of probiotics. Probiotics are living microorganisms that may exert health benefits upon ingestion. For example, they may help to balance disturbed intestinal microflora induced by weaning, control enteric diseases, and promote growth. The most commonly used probiotics for pigs include lactic acid-producing bacteria (LAB), (eg, Lactobacillus, Enterococcus, and Bifidobacterium species) and yeasts, particularly Saccharomyces species.1-4
As reviewed by Fairbrother et al5 and Conway,6 the effects on growth performance and pig health have been inconsistent when probiotics are added to dry diets. Freeze-dried probiotics can be administered in tablets or capsules, paste, powder, or granules, either administered directly or provided in feed.7,8 An alternative to delivering large numbers of LAB is the use of fermented feed, in which LAB are present as viable cells and additionally, metabolites produced during the fermentation process are included.9
Fermentation of feed protein may reduce protein quality and produce biogenic amines and ammonia, which may be toxic and negatively influence animal performance.10 Using carbohydrate-rich feedstuffs for fermentation may have a more favorable effect on growth of pigs than feeding complete compound diets.11 Whey is one of the most commonly used of a wide range of available co-products.12
Therapeutic fermented feeds contain LAB selected for properties enabling them to survive passage through the gastrointestinal tract.13-16 If fermentation of liquid feed for pigs could be successfully controlled by use of such bacterial inoculants, then the risk of postweaning coliform diarrhea might be reduced. This in turn would improve growth rate, feed efficiency, and well-being of weaned pigs. Bacterial inoculants could replace expensive organic acids, antibiotics, or both in weanling pig diets and contribute to efforts to reduce overall antimicrobial use in pigs.10,14
The main objective of this study was to examine the effect on growth performance, feed efficiency, and health (represented by diarrhea scores) of weanling pigs fed liquid diets containing fermented liquid whey plus dextrose (FLWD) inoculated with different species or strains of LAB. A second objective was to determine the fermentation dynamics of liquid whey-dextrose mixtures inoculated with different LAB by measuring the pH, dry matter (DM) content, and LAB counts of the whey-dextrose mixtures.
Material and methods
Experimental design
Two 21-day trials were conducted at the University of Guelph Swine Research Station. Each trial evaluated five dietary treatments (Table 1) which were fed from Day 0 to Day 21 of both trials. A total of 70 pigs per trial were ear tagged and weighed 1 day before weaning and were identified in each trial by weight and litter. On the day of weaning (Day 0), pigs were assigned to treatments, with littermates and mean initial body weights distributed evenly among treatments. Pigs were housed in two contiguous pens per treatment, with two contiguous pens left empty between treatment blocks. This study was approved by the University of Guelph Animal Care Committee and conducted according to the guidelines of the Canadian Council of Animal Care.17
Table 1: Five dietary treatments evaluated in a weanling pig performance study*
* Seventy pigs per trial (28 pigs per treatment) were weaned and assigned to treatments at approximately 3 weeks of age. Diets were fed for 21 days in each of two trials. † Composition of basal dry diet: Digestible energy, 13.69 mJ/kg; protein, 20.35%; fat, 4.86%; sodium, 0.24%; potassium, 0.81%; calcium, 0.78%; phosphorus, 0.63%; chloride, 0.42%; total lysine, 1.29%; digestible lysine, 1.17%; digestible methionine-cystine, 0.62%; digestible tryptophane, 0.22%; methionine, 0.34; cystine, 0.27%; whey, 7.5%. The dry diet fed to Group 5 was supplemented with growth-promoting levels of zinc (140 UI/kg) and copper sulphate (25 UI/kg). ‡ For Group 1, naturally fermented liquid whey-dextrose, and for Groups 2, 3, and 4, liquid whey-dextrose fermented with lactic acid bacteria, were mixed 1:1 with the basal diet just before feeding. |
Animals and housing
Seventy Yorkshire piglets were used in each trial, with an average weaning age of 19.3 ± 1.4 days in the first trial and 22.9 ± 2.8 days in the second trial, and an average body weight (BW) of 6.6 ± 0.99 kg in the first trial and 7.2 ± 0.86 kg in the second trial. Before weaning, piglets had free access to water but did not receive creep feed. Nursery pens had fully slatted floors and were equipped with feeders divided into four feeding places. The nursery room was emptied, cleaned, disinfected, and dried prior to use.
Experimental diets
The Group 5 feed was a dry mash (basal diet) supplemented with 0.1% lincomycin and growth-promoting levels of zinc oxide and copper sulfate (Table 1). Groups 1 through 4 were fed FLWD diets mixed with the basal diet without growth-promoting supplements (Table 1). For Group 1 pigs, the liquid whey-dextrose mixture was allowed to ferment naturally and then was mixed with the basal diet at a ratio of 1:1 just before feeding. Diets for Groups 2, 3, and 4 were prepared similarly, but bacterial inocula were added to the whey-dextrose mixture at the beginning of the fermentation step. For Group 2, the inoculum contained Lactobacillus rhamnosus (strain GR-1) and Lactobacillus reuteri (formerly Lactobacillus fermentum) (strain RC-14), both of human origin. These human probiotic strains have been well characterized, are tolerant to bile, and were selected as a result of extensive in vitro and human studies.18 The inocula for Groups 3 and 4 each contained three different isolates of Lactobacillus plantarum of pig origin (isolated from either nursing or weanling pigs). These six L plantarum strains were selected for their tolerance to pH 4 and 0.3% bile acids in laboratory studies and for their ability to inhibit different strains of enterotoxigenic Escherichia coli in vitro.19
Preparation of inocula for fermented liquid-whey diets
To prepare the inocula, the LAB were grown anaerobically at 37°C for 48 hours on Mann-Rogosa-Sharpe (MRS) agar plates (Oxoid, Baisngstoke, UK). A McFarland 3 suspension (approximately 9 × 108 colony forming units [CFU] per mL) was prepared in sterile phosphate buffered saline (PBS; pH 7.4). Three mL of each suspension was used to inoculate 27 mL of sterile MRS broth and this was incubated aerobically for 48 hours. The inocula were prepared individually for each LAB.
Preparation of fermented liquid whey-dextrose
The four different liquid whey-dextrose mixtures were prepared using 20-L plastic storage jugs. In each jug, nonhygroscopic whey powder (Pestell, New Hamburg, Ontario, Canada; 2.5 kg) and dextrose (Cerelose; Corn Products US, Westchester, Illinois; 2.5 kg) were mixed with distilled water (15 L) for 15 minutes. Three jugs were inoculated with their respective LAB (Groups 2, 3, and 4; 25 mL per jug). After inoculation, jugs were rotated by hand for 10 minutes to mix the contents. A fourth jug of liquid whey-dextrose was not inoculated with LAB. The four jugs were incubated at 37°C for 5 days, then stored at 4°C at the farm until fed. Four batches of FLWD mixtures were prepared for each treatment in each trial. Feeding of each batch began on the last day of fermentation.
Testing for pH, DM, and LAB counts in liquid-whey mixtures
Immediately after the liquid-whey mixtures were inoculated with LAB and then on each of the 5 days of incubation and the first 2 days of storage, a 20-mL sample was collected from each FLWD for determination of pH, DM content, and LAB counts Immediately before sampling, the liquid whey was stirred for at least 5 minutes. The pH of the mixture was measured daily using a pH meter (Accumet AR15; Fisher Scientific Company, Pittsburg, Pennsylvania). Samples were stored at -20°C until the end of each trial when all samples were thawed at room temperature and tested as a batch. A pooled sample was prepared for the four batches of each FLWD and tested in duplicate for DM content by drying in an oven at 103°C for 24 hours. To determine the numbers of CFU per mL of FLWD mixture, serial 10-fold dilutions of the liquid whey were made in PBS (pH 7.2). Aliquots of the 106 to 109 dilutions were inoculated onto MRS agar and incubated anaerobically at 37°C for 48 hours. Colonies were counted on each plate, and the concentrations per mL of mixture were calculated and expressed as base 10 logarithms.
Testing pig-origin LAB strains for acid and bile tolerance and inhibitory activity against Escherichia coli
LAB isolates were cultured on MRS agar. Colonies were suspended in 10 mL of PBS (pH 7.2) to achieve a concentration approximating a McFarland 2 suspension (6 × 108 CFU per mL). One mL of suspension was cultured either in unsupplemented MRS broth (control) or in MRS broth containing HCl (pH 2 and pH 4) or dried unfractionated bovine bile (Sigma Chemical Company; 1.5 g and 3Â g of bile per mL of MRS to prepare broth containing 0.15% and 0.30% bile, respectively). Optical absorbance (OD620) was determined at the time of inoculation and after aerobic culture at 35°C for 24 hours, and changes in absorbance were calculated. Tolerance to pH or bile was calculated as the percentage of growth of LAB in each MRS medium compared to growth in the control broth. Isolates were considered resistant to acid or bile when the OD620 of the supplemented MRS broth after 24 hours culture was ≥ 90% of that of the control broth; moderately resistant when the OD620 was ≥ 70% but < 90% of that of the control broth; slightly resistant when the OD620 was ≥ 40% but < 70% of that of the control broth; and poorly resistant when the OD620 was < 40% of that of the control broth.
Inhibitory activity of the isolates against E coli was determined by evaluating the effect of cell-free LAB culture supernatant on the growth rate of an O149:F4-positive E coli strain (JG-280). Briefly, LAB strains were cultured at 37°C in MRS broth in an anaerobic environment, then centrifuged at 3800g for 10 minutes at 4°C. Supernatant was passed through a 0.2-μm syringe filter (Fisher Scientific Ireland Ltd, Dublin, Ireland). A control was prepared by adjusting MRS broth to the pH of the supernatant with HCl. Supernatants were frozen at -80°C for 24 hours and then concentrated 10-fold by freeze drying (Freeze Drying System; Thermo Savant, Farmingdale, New York) for 36 to 48 hours. Escherichia coli strains were cultured aerobically in duplicate on blood agar for 24 hours at 37°C. A McFarland 2 suspension was prepared and 100 μL of each E coli suspension was diffusely inoculated onto tryptic soy agar (TSA) with a swab. Six 6-mm diameter full-thickness wells were cut out of each agar plate. Freeze-dried LAB were reconstituted with sterile water to one tenth of the original volume (approximately 700 μL). Seventy μL of a LAB suspension was placed in each well. Reference LAB strains included an equine-origin strain, Lactobacillus pentosus WE720 (pH 3.7), and a human-origin strain, L rhamnosus GG18 (pH 3.7). Following aerobic incubation of the TSA plates at 37°C for 24 hours, the diameter of the zone of inhibition around each well was measured.
Growth-parameter calculations
Pigs were individually weighed weekly (Days -1, 7, 14, and 21) on an electronic scale accurate to 0.1 kg, and ADG was calculated per week. The liquid whey-dextrose mixed with the dry feed was offered once a day to the pigs, and feed remaining was removed the following day prior to offering fresh feed. Feed intake (disappearance or usage) per day per pen was calculated as the weight of the feed offered minus the weight of the feed remaining in the feeder. Although feed wastage through the floor slats was observed, this was not taken into account to calculate daily feed intake. The approximate DM in the feed offered to the pigs was calculated daily by adding the DM content of the liquid whey at day 5 of fermentation and the DM of the dry feed, assuming an average DM of 88% for the dry feed. The DM of unconsumed feed was similarly calculated daily assuming a ratio of 1:1 of liquid whey-dextrose and dry feed remaining in the feeder.
The feed-to-gain (F:G) ratio was calculated at pen level as average daily feed intake (ADFI) divided by mean ADG per pen and expressed as kg of feed per kg of body weight gained.
Fecal consistency was evaluated for each pig daily by a person blinded to treatment. The following criteria were used: 0 = firm dry feces; 1 = soft and pasty feces; 2 = yellowish fluid feces; 3 = clear water-like feces.
Culture of rectal swabs for E coli
Rectal swabs were collected from each pig on Days 0 and 14 and cultured on MacConkey agar and sheep blood agar. Suspected hemolytic E coli colonies on the blood agar plates were considered for further investigation. The slide agglutination test for F4 and O149:K91 antigens was performed for each hemolytic isolate using standard techniques.21
Necropsy procedures
Three pigs on Day -1, and one pig on each of Days 7 and 21, were euthanized by IV injection of sodium pentobarbital (Euthanasol; Schering-Plough Animal Health, Division of Schering Canada, Pointe Claire, Quebec, Canada) for culture of intestinal content. On Day -1, a convenience sample of three pigs was selected from the farrowing room for euthanasia. On Day 7, the pig with the median body weight in each pen was selected. On Day 21, with an uneven number of pigs in each pen, a random table was used to select one of the two pigs ranked in the median body weight for the pen. Weaned pigs were euthanized 24 hours after their last meal. Samples of stomach, small and large intestine, and cecal contents were obtained from each pig for pH determination (Days -1, 7, and 21) and LAB counts (Days -1 and 7). For each sample type, pH was determined within 1 hour of sample collection using a pH metre (Accumet Research AR15). Necropsies were performed on all pigs that died or were euthanized during the trial. Qualitative evaluations of the pig’s intestinal tract contents and body condition were performed at post mortem.
Statistical analysis
The association between treatments and pH, LAB counts, and DM content of the FLWD were analyzed in a mixed model with repeated measures and taking into consideration the fixed effects of treatment and day of fermentation. The crossed effects of trial and treatment were considered random effects. Analysis was performed in SAS Version 9.1 (PC/SAS Institute Inc, Cary, North Carolina). A post hoc comparison test (preplanned t test) was performed to identify treatment groups that differed significantly. A log 10 transformation was performed on LAB counts in order to approach a normal distribution. Results were expressed as mean ± standard error (SE).
The ADG, ADFI, and F:G ratio at pen level were subjected to analysis of variance using the General Linear Model of SAS. For ADG, ADFI, and F:G ratio, the model included trial number, treatment, and the repeated effect of week with all possible interactions. Where treatment was significant, a post hoc comparison test (preplanned t test) was performed to identify treatments that differed significantly.
Results of testing the pH of the stomach, small intestine, cecum, and colon contents were analyzed using the Mixed procedure in SAS. The models included treatment and the repeated effect of week. Trial was removed from the model of the pH of stomach, small intestine, cecum, and colon contents because of the small sample size. Results are expressed as least squares means ± SE.
Diarrhea scores by treatment were analyzed by the Kruskal-Wallis one-way nonparametric test. Sum ranks of the treatment groups were compared using the Wilcoxon rank sum test.
Descriptive statistics were used for mortality, and treatment groups were tested for association with hemolytic E coli-positive culture using a chi-square test. These latter analyses were performed in Stata (Intercooled Stata 8 for XP, 2003; Stata Corporation, College Station, Texas).
Results
Characteristics of pig-origin LAB
The LAB of pig origin selected for this experiment showed moderate growth in broth at pH 4 and in 0.15% bile (70% to 90% of growth in control broth). Growth in 0.30% bile ranged from slight to moderate (42% to 85% of growth in control broth). All isolates were poorly resistant to pH 2 (< 7% of growth in control broth).
The diameter of the zone of inhibition for the pig-origin LAB was >15 mm (range 15.7 to 17.5 mm for the six strains). The average zones of inhibition for the reference strains were 13.7 ± 0.11 mm (L rhamnosus GG) and 15.0 ± 0.4 mm (L pentosus WE7).
Changes in pH, DM, and LAB counts in fermenting liquid-whey mixtures
The pH and DM of the liquid whey-dextrose mixtures decreased in all treatments during fermentation (P < .001) (Table 2) and there was a treatment effect on pH (P < .01). Immediately after fermentation began and one day later, pH differences were not observed among the liquid whey-dextrose mixtures. On the second day of fermentation, the pH of the Group 2 whey-dextrose mixture was lower than that of the Group 1 (naturally fermenting) mixture (PÂ = .04) and tended to be lower than those of the Group 3 and 4 liquid mixtures (P = .08). No differences were observed for other days. A pH value of ≤ 4 was reached on the third day of fermentation in the Group 2 and 3 liquid whey-dextrose mixtures, on the fourth day of fermentation in the Group 4 mixture, and on the fifth day of fermentation in the Group 1 mixture.
Table 2: Mean (± SE) of pH, dry matter (DM) content, and counts of lactic acid bacteria (LAB) in three liquid fermented whey-dextrose mixtures inoculated with different LAB (Groups 2, 3, and 4) and in one naturally fermented whey-dextrose mixture (Group 1)*
* Liquid whey-dextrose mixtures were incubated at 37°C for 5 days, then stored at 4°C. The first samples were collected immediately after inoculation with LAB. Treatment groups are described in Table 1. † After controlling for time in the Mixed model, pH differed among groups over time (P < .001). Differences were observed between Groups 1 and 2 (P < .001), between Groups 1 and 3 (P < .01), and between Groups 2 and 4 (P < .01). The pH tended to differ (P < .10) between Groups 1 and 4 and between Groups 2 and 3. ‡ After controlling for time in the Mixed model, DM did not differ among treatments over time. § After controlling for time in the Mixed model, LAB log 10 differed among Groups over time (P < .001). Differences were observed between Group 1 and Groups 2, 3, and 4 (P < .001). abcd For each parameter, values with different superscripts within a column differ significantly (P < .05; pre-planned t test). ND = not done. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The log 10 LAB count was associated with treatment (P < .001) and day of fermentation (P < .001). On the day of LAB inoculation, the log 10 LAB count was significantly lower for the Group 1 fermentation mixture than for the Group 2, 3, and 4 mixtures (P < .01), and increased in all five mixtures during the first 24 hours of fermentation. No differences in log 10 LAB counts were observed among fermentation mixtures during the remaining 5-day fermentation period (Table 2).
Growth parameters
The least squares means for ADG, ADFI, and F:G ratio are presented in Table 3. The ADG model showed a significant effect of trial (P < .001), treatment (P < .01), and week (P < .001). The interaction of treatment and week was also significant (P < .01); therefore, ADG data were analyzed within week. Differences in ADG were observed in the third week of Trial 1. Group 5 ADG was higher than that of the other groups (P < .01). Moreover, ADG was higher in Groups 3 and 4 than in Group 2 (P = .03 and P = .02, respectively). In the ADFI model, trial, treatment, and week and the interaction of trial*week and treatment*week were significant effects (P < .001). The three-way interaction of treatment, trial, and week was significant (P = .02). Analysis within week and trial showed that ADFI at Week 3 was higher in Group 5 than in the other groups in both Trials 1 and 2 (P = .03 and P = .04, respectively).
Table 3: Average daily gain (ADG), average daily feed intake (ADFI), and feed:gain (F:G) (least squares means) for groups of pigs weaned at approximately 3 weeks of age (Day 0) and fed four different fermented liquid whey-dextrose diets and a conventional dry diet*
* Diets described in Table 1. Pigs were weighed the day before weaning (Day –1) and weekly thereafter. † Calculated on dry matter basis (kg/day) ‡ F:G calculated as ADFI (dry matter basis) ÷ ADG ab Within a row, means with no common superscript differ (P < .05; pre-planned t test when treatment was significant [P < .05] in the general linear model). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
On some days, pigs receiving the fermented liquid diets ate all the feed available and had to wait for the feeder to be filled, whereas the pigs receiving the dry feed were fed ad libitum. This feed-intake restriction was especially pronounced in the third week of both trials. No significant effects of treatment and trial were observed for F:G, but week was significant (P = .01). Analysis within week and trial showed that in Trial 1, F:G at Week 3 was higher in Group 2 than in Groups 4 and 5. However, variation in F:G in Group 1 was high in the first and second weeks of Trials 1 and 2 (data not shown).
Mortality, culture of hemolytic E coli, and diarrhea scores
The proportion of swabs positive for hemolytic E coli on Days 0 and 14 are presented in Table 4. Two pigs died in each of Groups 1, 3, and 4; one pig died in Group 5; and no pigs died in Group 2. In each of Groups 3 and 4, one pig died due to diarrhea. In Group 1, two pigs were euthanized because of anorexia, dehydration, and severe diarrhea. No mortality due to diarrhea was observed in Groups 2 and 5. Some pigs were euthanized for reasons other than diarrhea: one pig for lameness in Group 5, one pig because of meningitis in Group 3, and one pig because of respiratory problems in Group 4.
Table 4: Proportion of rectal swabs positive for hemolytic Escherichia coli on Days 0 and 14 among five groups of pigs weaned on Day 0 and fed dry feed or liquid-whey mixtures with or without lactic acid bacteria*
* Diets described in Table 1. † E coli-positive = rectal swabs cultured positive for hemolytic E coli. ab Percentage values with no common superscript within a column differ (P < .05; chi-square). |
|||||||||||||||||||||||||
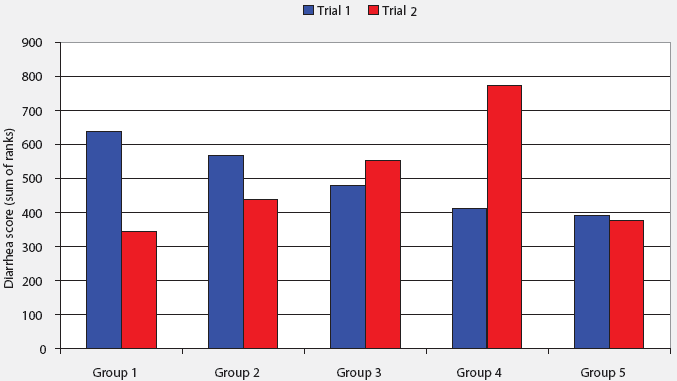
No significant differences were observed in diarrhea scores among treatment groups in the first trial. In the second trial, the average diarrhea score was higher in Group 4 than in any other treatment group (PÂ <Â .001). Average diarrhea scores combined for both trials were higher in Group 4 than in Group 5 (P = .05) (Figure 1). The O149:K91:F4 E coli that is commonly associated with postweaning colibacillosis was not recovered from rectal swabs in these trials. However, other strains of hemolytic E coli were identified. No significant differences among treatment groups were observed at Day 0. The proportion of rectal swabs positive for hemolytic E coli was higher at Day 14 for Group 5 than for Group 4 (P < .01; Table 4).
| Figure 1: Average diarrhea score by trial among
groups of pigs fed four fermented liquid whey-dextrose diets and a conventional
dry diet containing an antimicrobial (treatments described in Table 1).
Diarrhea scores for each 21-day trial are expressed as sum of ranks arranged
in order of magnitude. Scoring system: 0, firm dry feces; 1, soft pasty
feces; 2, yellowish fluid feces; 3, clear water-like feces. Sum of diarrhea
scores per pig were analyzed by the Kruskal-Wallis one-way ANOVA. No treatment
effects were observed (P > .10) in Trial 1. In Trial 2, Group
4 differed from all other treatment groups (P <
.001).
|
Post-mortem observations and intestinal content pH
Pigs fed the fermented liquid whey-dextrose diets generally had distended stomachs and large ceca filled with gas and liquid contents. In two emaciated Group 1 pigs that died, the gastric mucosa was dark brown, probably due to gastric ulcers.
There were no significant differences in pH of stomach, small intestinal, or cecal contents at Day 7 compared to Day -1, although the pH of the large intestinal contents of all groups was lower on Day 7 than on Day -1 (P = .03). However, the pH of stomach (P < .001), small intestinal (P <Â .01), and cecal (P < .01) contents decreased between Day 7 and Day 21 in all treatment groups.
Discussion
To our knowledge, this is the first published study evaluating the fermentation dynamics of liquid whey-dextrose inoculated with LAB and reporting the effect on growth performance and health when this fermented product is fed to weanling piglets. Fermentation was better when whey-dextrose mixtures were inoculated with LAB. Decreasing DM and pH of the FLWD indicated that lactic acid, volatile fatty acids, and alcohol were produced, likely by LAB or yeast.11,14,22 A low pH in combination with high concentrations of organic acids in the feed are likely to be important for inhibition of pathogenic bacteria, and there is evidence that the fermentation process is faster and more controlled when specific LAB are used, minimizing formation of undesirable fermentation products.10,11,16,23 The fermentation procedure used in this experiment was highly controlled and may not be practical on commercial farms. In addition, development of this procedure for farm environments may be limited by the time needed to grow LAB for the fermentation process on the farm. However, one study performed on a farm is reported,24 in which pre-fermented feed and L plantarum were added to a fermented liquid feed. A stable environment was established from the first day of fermentation, with high levels of LAB, controlled yeast populations, and reduction of Enterobacteriaceae. In contrast, in nonfermented liquid feed, natural LAB populations were not stabilized for 10 days, there was an undesirable peak of yeast during the first week, Enterobacteriaceae were very unstable, and addition of organic acids was required to lower the pH.24
While laboratory testing can provide information useful for selection of potentially effective probiotic strains, effects on intestinal microflora can be accurately determined in vivo25 only by the effect on the growth performance and health of the pig.8 The results of the present study demonstrate that FLWD inoculated with probiotic mixtures show promise in reducing numbers of enteric bacteria in pigs. However, this study lacked sufficient sample size and study power to detect a difference in parameters such as mortality. Although mortality did not differ significantly among treatment groups, the rate of 7% mortality observed in Group 1 (fed fermented feed not inoculated with LAB), would be unacceptable in a commercial herd. In addition, as the feeding procedure was performed manually, availability of feed in the third week of both trials differed between the groups on fermented liquid feed and the group on dry feed. Pigs receiving the fermented liquid feed ate all the feed available and had to wait for the feeder to be filled, whereas the pigs receiving the dry feed were fed ad libitum. Therefore, differences among groups fed dry feed and fermented liquid feed in the third week must be interpreted with caution. A larger trial with a feeder system allowing ad libitum access to the fermented liquid feed would be needed to specifically assess the effect of FLWD on mortality and growth performance.
The two human-origin LAB (L rhamnosus GR-1 and L reuteri [formerly fermentum] RC-14) used in this experiment have proven to have desirable properties and clinical effects in humans4,13,26 and rats.27 Further research is needed to determine the effect of these human-origin bacteria on the growth performance and health of pigs. The six L plantarum swine-origin strains used in these trials had shown probiotic potential in vitro, ie, they were able to inhibit growth of enteropathogenic E coli and survive passage through the intestinal tracts of pigs.19 In these trials, the L plantarum strains showed promise in terms of improving ADG and ADFI, but their effect on lowering diarrhea rates was inconsistent. Further studies are needed, perhaps combining L plantarum strains with a strain such as L reuteri, known to propagate in pigs.28
Current data on the effects of feeding fermented diets to weanling piglets are based primarily on research using completely fermented compound diets.10,29 However, such diets are reported to have an undesirable effect on F:G ratio, especially in weanling pigs.9,10,30 The results of a limited number of studies show favorable ADG and F:G ratios in pigs fed fermented co-products11,29 compared to pigs fed nonfermented liquid diets. The Scholten study9 is limited in that it compared feeding fermented wheat with nonfermented wheat, but did not include a dry-feed control.
In our study, no significant differences in ADG were observed among treatments during the first 2 weeks. This result agrees with the studies of Scholten (2001)9 and Lawlor et al (2002)10 in which pigs were fed fermented complete compound diets or a dry diet, and a fermented liquid wheat and nonfermented liquid wheat diet, respectively. However, in the present study, differences in ADG were observed or tended to be observed in the third week. In general, ADG was better in pigs receiving a conventional dry diet containing an antibiotic (Group 5) than in pigs fed the partly fermented liquid diets. In addition, pigs fed the FLWD consumed more feed than the Group 5 pigs during the first 2 weeks of the trial. However, in the third week, pigs fed the dry feed ate more than the pigs fed the fermented liquid diets because of unintentional restriction of the liquid feeds. The consequences of this semi-restricted feeding might be a lower ADG in pigs on liquid feed compared to those with ad libitum access to feed. We assume that if the fermented feed had been offered ad libitum, the pigs would have had a higher average daily intake, resulting in a better daily gain. Likewise, restricted feeding might affect the F:G ratio. It was noted that F:G ratios were similar in groups receiving the fermented liquid diets with inocula of pig origin and the ad libitum dry feed group. Restricted feeding can improve feed efficiency, which may account for the apparently better F:G in some groups of pigs fed fermented liquid feeds with LAB.
The high F:G ratio observed for animals fed the fermented liquid feed without LAB may have been a result of feed wastage or disease.10 Feed wastage is very difficult to measure,31 and the terms “feed usage” or “feed disappearance” should be applied to liquid feeding instead of “feed intake.”10 It is possible that groups fed fermented liquid feeds without LAB wasted more feed because it was unpalatable. Squire32 showed that levels of acetic acid were higher when corn distillers solubles were fermented without inoculants than when corn distillers solubles were fermented with inoculants. High levels of acetic acid in swine liquid feeds have been associated with poor feed intake due to the vinegar flavor and pungent odour.33
Failure to gain weight because of disease is another possible explanation for the high F:G ratios in pigs on the fermented liquid diets. Liquid fermented diets can cause ulceration in the esophageal region of the stomach, possibly due to high levels of volatile fatty acids entering epithelial cells causing acidification, swelling, inflammation, and ulceration.34 Gastric lesions were present only in the pigs fed the fermented feed without LAB inoculum, and this may be related to the type of LAB that grew in this feed and the acids produced during the fermentation process.33 Superficial and deeply penetrating gastric lesions were observed in a study in which young gnotobiotic pigs were fed high-carbohydrate diets inoculated with fermentative commensal Lactobacillus and Bacillus strains.34
There is evidence in the present study that some mixed cultures of LAB, in particular strains GR-1 and RC-14, reduced fecal E coli in the pigs. This was observed on Day 14 of the trial. Gardiner et al25 reported lower counts of enteric bacteria in pigs on days 15, 22, and 26 of probiotic consumption than prior to or during the first week of culture consumption (days 3 to 8). Similar observations were reported by Canibe and Jensen35 in growing pigs. The significant increase in hemolytic E coli found in pigs fed antibiotics might be expected since lincomycin is effective against gram-positive bacteria,36 including normal intestinal microbiota, establishing conditions that permit invasion by pathogens. This finding is a concern, as gram-negative pathogens have adverse effects not only on the pigs, but also on consumers if there is contamination at slaughter.37 The ability of LABs to decrease enteric bacterial counts in pigs may be viewed as a more important outcome than the weight gain obtained with antibiotic use. A longer-term study would determine the extent to which weight gains truly differ between probiotic-fed and antibiotic-fed animals, and an economic analysis could then assess the impact to the producer.
Although some studies have been published concerning the ability of probiotics and fermented feed to reduce levels of enteric bacteria in the GI tracts or feces of pigs,35,38-41 evidence for the effectiveness of fermented liquid feed diets as anti-diarrhea agents has not been tested. In the present study, pigs in all treatment groups developed diarrhea. The incidence of diarrhea was highest in the control group and one of the groups inoculated with pig-origin LAB (Group 4). Danish veterinarians29 recommend feeding newly weaned pigs dry pelleted feed for at least the first week after weaning before a fermented feed is offered. Liquid feeding of newly weaned pigs is a challenge, as intake during the first 7 to 14 days post weaning is highly variable. Build-up of liquid feed in the troughs must be controlled to avoid feed spoilage, loss of feed palatability, growth of unfavorable microbes in the troughs, and resulting compromised pig performance. This is one of the main reasons that use of dry feed during the first 1 to 2 weeks post weaning is a common practice in Europe. In addition, feeding fresh liquid whey with high levels of lactose may increase diarrhea, perhaps because of the laxative effect of the sugar.8,41,42 It is possible that during fermentation in the present study, lactose was not completely degraded to lactic acid in some of the liquid-whey batches, and high levels of lactose were still present in the liquid whey, producing diarrhea in the pigs.
Due to time restrictions in this study, it was not possible to determine the occurrence of LAB in the intestinal contents of the pigs. In one report,24 strains of L plantarum inoculated into fermented liquid feed were recovered from the feces of pigs, indicating that the microflora of the feed might influence the intestinal microflora of the weaned pigs. More studies are needed to determine whether the LAB used in this study can influence the intestinal microflora of pigs. In agreement with previous reports,11,38 a decrease of pH in stomach, small intestinal, and cecal contents over time was observed. Mikkelsen and Jensen38 and Scholten et al11 demonstrated lower gastric pH and higher gastric lactic acid content in piglets fed fermented liquid diets 14 days and 28 days after weaning, respectively. Low intestinal pH might be associated with the smaller numbers of E coli found in the pigs in our study until Day 21 of sampling.
In other studies, no significant differences were observed in the pH of stomach and small intestinal content in pigs fed fermented or nonfermented diets.11,35,40 In the present study, the pigs were fed for the last time 24 hours before they were euthanized. At the time of death, the pigs may not have had lactic acid from the feed in their stomachs, and this would be reflected in a higher gastric pH.35 In addition, it is reported that organic acids are rapidly absorbed by intestinal mucosal cells,9,33 and by the time the pigs were euthanized, most of the lactic acid ingested in the feed might already have been taken up by intestinal cells. In addition, it is likely that the tendency for a higher pH in the small intestinal content of pigs fed the fermented liquid whey at Day 21 compared to those fed the dry feed might be associated with the presence of lactic acid increasing the volume as well as the protein and bicarbonate content of pancreatic secretion, which can buffer the pH in the first part of the small intestine.11
Larger repeated controlled studies with different inclusion rates of the fermented liquid whey with dry feed must be conducted to fully understand the effects and interactions of different factors that may occur when probiotics and fermented feeds are used in weaned pigs. In addition, in vivo trials in pigs inoculated with a known pathogenic E coli might provide further information about the ability of the probiotic cultures investigated in the present study to reduce intestinal pathogens.
Implications
- Fermented liquid whey-dextrose inoculated with LAB may reduce shedding of hemolytic E coli in weaned pigs, but not the prevalence of diarrhea.
- Positive effects on growth performance may be observed when pigs are fed liquid whey-dextrose products fermented with inoculated strains of LAB compared to whey-dextrose products allowed to ferment spontaneously.
- Further experiments using fermented whey-dextrose products as feed for newly weaned pigs are needed to determine effects on the health of the pigs.
Acknowledgements
We want to thank the Ontario Research and Development Challenge Fund and the Ontario Ministry of Agriculture, Food and Rural Affairs for their financial support. We also want to thank Dr Gregor Reid for providing the human strains of lactic acid bacteria and Dr Carlton Gyles (Department of Pathobiology, University of Guelph, Ontario, Canada) for providing the E coli strain JG-280.
References
1. Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol. 2000;78:80–88.
2. Rolfe RF. The role of probiotic cultures in the control of gastointestinal health. J Nutr. 2000;130:396S–402S.
3. Holzapfel WH, Haberer P, Geisen R, Bjorkroth J, Schillinger U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am J Clin Nutr. 2001;73:365S–373S.
4. Reid G, Friendship R. Alternatives to antibiotic use: microbiological perspective. Anim Biotech. 2002;13:97–112.
5. Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial type, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005; 6:17–39.
*6. Conway PL. Specifically selected probiotics can improve health and performance of pigs. In: Cranwell PD, ed. Manipulating Pig Production VII. Proc Australasian Pig Sci Assoc Conf. Adelaide, South Australia. 1999:220–224.
*7. Alm L. The effect of Lactobacillus acidophilus administration upon the survival of Salmonella in randomly selected human carriers. Prog Food Nutr Sci. 1983;7:13–17.
8. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J Nutr. 1994;125:1401–1412.
9. Scholten RHJ. Fermentation of liquid diets for pigs [PhD thesis]. Wageningen Netherlands: Wageningen Universiteit; 2001.
10. Lawlor PG, Lynch PB, Gardiner GE, Caffrey PJ, O’Doherty JV. Effect of liquid feeding weaned pigs on growth performance to harvest. J Anim Sci. 2002;80:1725–1735.
11. Scholten RHJ, van der Peet-Schwering CMC, den Hartog LA, Balk M, Schrama JW, Verstegen MWA. Fermented wheat in liquid diets: Effects on gastrointestinal characteristics in weanling piglets. J Anim Sci. 2002;80:1179–1186.
*12. de Lange C, Braun K, Squire J, Friendship R, Amezcua R, Farzan V. Swine liquid feeding: Research update. Fine tuning technology to meet the needs of tomorrow. Proc Swine Liquid Feeding Assoc. Stratford, Ontario. 2004.
13. Alvarez-Olmos MI, Oberhelman RA. Probiotic agents and infectious diseases: a modern perspective on a traditional therapy. Clin Infect Dis. 2001;32:1557–1576.
14. Geary TM, Brooks PH, Beal JD, Campbell A. Effect on weaner pig performance and diet microbiology of feeding a liquid diet acidified to pH 4 with either lactic acid or through fermentation with Pediococcus acidilactici. J Sci Food Agric. 1999;79:633–640.
15. van Winsen RL, Lipman LJA, Biesterveld S, Urlings BAP, Snijders JMA, van Knapen F. Mechanism of Salmonella reduction in fermented pig feed. J Sci Food Agri. 2000;81:342–346.
16. Demecková V, Kelly D, Coutts AGP, Brooks PH, Campbell A. The effect of fermented liquid feeding on the faecal microbiology and colostrum quality of farrowing sows. Int J Food Microbiol. 2002;79:85–97.
17. University of Guelph Animal Care Policy and Procedures. Revisions approved by Senate Research Board. Available at: http://www.uoguelph.ca/research/policies/Adobe/AnimalCarePolicy.pdf. Accessed 22 August 2007.
18. Gardiner GE, Heinemann C, Baroja ML, Bruce AW, Beuerman D, Madrenas J, Reid G. Oral administration of the probiotic combination Lactobacillus rahmnosus GR-1 and L. fermentum RC-14 for human intestinal applications. Int Dairy J. 2002;12:191–196.
19. Amezcua MDR. Post-weaning diarrhea caused by Escherichia coli: Prevalence, antibiotic resistance, investigation of risk factors and control methods [PhD thesis]. Guelph, Ontario, Canada: University of Guelph, 2005.
20. Weese JS, Anderson MEC, Lowe A, Penno R, Da Costa TM, Button L, Goth KC. Screening of the equine intestinal microflora for potential probiotic organisms. Equine Vet J. 2004;36:351–355.
21. Edwards PR, Ewing WH. The genus Escher-ichia coli. In: Identification of Enterobacteriaceae. 3rd ed. Minneapolis, Minnesota: Burgess Publishing; 1972:67–107.
*22. Smits B. Chemical composition, digestibility and nutritive value of high moisture by-products in pig nutrition. Proc 49th Ann Meet Eur Assoc Anim Prod. Warsaw, Poland. March, 1998.
23. van der Wolf PJ, Wolbers WB, Elbers ARW, van der Heijden HMJF, Koppen JMCC, Hunneman WA, van Schie FW, Tielen MJM. Herd level husbandry factors associated with the serological Salmonella prevalence in finishing pig herds in the Netherlands. Vet Microbiol. 2001;78:205–219.
24. Pumed-Ferrer C, Kivelä I, Hyvonen P, von Wright A. Survival, growth and persistence under farm conditions of a Lactobacillus plantarum strain inoculated into liquid pig feed. J Appl Microbiol. 2005;99:851–858.
25. Gardiner GE, Casey PG, Casey G, Lynch PB, Lawlor PG, Hill C, Fitzgerald GF, Stanton C, Ross RP. Relative ability of orally administered Lactobacillus murinus to predominate and persist in the porcine gastrointestinal tract. Appl Environ Microbiol. 2004;70:1895–1906.
26. Reid G. The scientific basis for probiotic strains of Lactobacillus. Appl Environ Microbiol. 1999;65:3763–3766.
27. Anukam KC, Osazuwa EO, Reid G. Improved appetite of pregnant rats and increased birth weight of newborns following feeding with probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus fermentum RC-14. J Appl Res. 2005;5:46–52.
28. Rodriguez E, Arques JL, Rodriguez R, Nunez M, Medina M. Reuterin production by lactobacilli isolated from pig faeces and evaluation of probiotic traits. Lett Appl Microbiol. 2003;37:259–263.
*29. Pedersen AØ. Fermented liquid feed for weaners and pigs. Int Pig Topics. 2003;18:7–9.
30. LeDividich J, Sève B. Effects of underfeeding during the weaning period on growth, metabolism, and hormonal adjustments in the piglet. Domestic Anim Endocrinol. 2000;19:63–74.
31. Patience JF, Thacker PA, de Lange CFM. Feeding management of market hogs. In: Swine Nutrition Guide. 2nd ed. Saskatoon, Saskatchewan: Prairie Swine Centre Inc; 1995.
32. Squire J. Fermentation of an alternative feedstuff for use in swine liquid feeding [MSc thesis]. Guelph, Ontario, Canada: University of Guelph; 2004.
*33. Mroz Z. Organic acids of various origin and physico-chemical forms as potential alternatives to antibiotic growth promoters for pigs. Proc Int Symp Dig Physiol Pigs. Banff, Alberta. 2003:Vol 1.
34. Krakowka S, Eaton KA, Rings DM, Argenzio RA. Production of gastroesophageal erosions and ulcers (GEU) in gnotobiotic swine monoinfected with fermentative commensal bacteria and fed high-carbohydrate diet. Vet Pathol. 1998;35:274–282.
35. Canibe N, Jensen BB. Fermented and nonfermented liquid feed to growing pigs: effects on aspects of gastrointestinal ecology and growth performance. J Anim Sci. 2003;81:2019–2031.
36. Ahrens FA. Antimicrobial drugs. In: Dyer D, Hsu W, Riedesel D, Ware W, eds. The National Veterinary Medical Series. 1st ed. Baltimore, Maryland: Lippincott Williams & Wilkins; 1996:207–228.
37. Grugel C, Wallmann J. Antimicrobial resistance in bacteria from food-producing animals. Risk management tools and strategies. J Vet Med B Infect Dis Vet Pub Health. 2004;51:419–421.
*38. Mikkelsen LL, Jensen BB. Effect of fermented liquid feed on the activity and composition of the microbiota in the gut of pigs. Proc Ann Meet Eur Assoc Anim Prod. Warsaw, Poland. 1998;263.
39. Chang YH, Kim JK, Kim HJ, Kim WY, Kim YB, Park YH. Selection of a potential probiotic Lactobacillus strain and subsequent in-vivo studies. Antonie van Leeuwenhoek. 2001;80:193–199.
40. van Winsen RL, Urlings BAP, Lipman LJA, Snijders JMA, Keuzenkamp D, Verheijden JHM, van Knapen F. Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Appl Environ Microbiol. 2001;67:3071–3076.
41. Højberg O, Canibe N, Knudsen B, Jensen BB. Potential rates of fermentation in digesta from the gastrointestinal tract of pigs: Effect of feeding fermented liquid feed. Appl Environ Microbiol. 2003;69:408–418.
42. Maswaure SM, Mandisodza KT. An evaluation of the performance of weaner pigs fed diets incorporating fresh sweet liquid whey. Animal Feed Sci Tech. 1995;54:193–201.
* Non-refereed references.