Original research |
Peer reviewed |
Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections
Muestras de fluido oral para el monitoreo de cerdos comerciales en crecimiento contra las infecciones causadas por el virus del síndrome reproductivo y respiratorio porcino y el circovirus porcino tipo 2
Utilisation d’échantillons de fluide oral pour la surveillance d’infections par le virus du syndrome respiratoire et reproducteur porcin et le circovirus porcin de type 2 chez des porcs en croissance
John R. Prickett; Wonil Kim, DVM, PhD; Robert Simer, DVM; Kyoung-Jin Yoon, DMV, PhD; Jeff Zimmerman, DVM, PhD
JRP, WK, KJY, JZ: Department of Veterinary Diagnostic and Production Animal Medicine, College of Veterinary Medicine, Iowa State University, Ames, Iowa. RS: Simer Veterinary Services, Axtell, Texas. Corresponding author: Dr Jeff Zimmerman, 2655 Veterinary Diagnostic Laboratory, College of Veterinary Medicine, Iowa State University, Ames, IA 50011-1250; Tel: 515-294-1073; E-mail: jjzimm@iastate.edu.
Cite as: Prickett JR, Kim W, Simer R, et al. Oral-fluid samples for surveillance of commercial growing pigs for porcine reproductive and respiratory syndrome virus and porcine circovirus type 2 infections.J Swine Health Prod. 2008;16(2):86–91.
Also available as a PDF.
SummaryObjectives: To validate the use of oral fluids to detect infections with porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2) in three commercial swine herds. Materials and methods: Oral-fluid and serum samples were collected from one barn on each of three PRRSV-infected finishing sites. Six pens per barn (20 to 30 pigs per pen) were sampled repeatedly, beginning when the pigs entered the facilities (3 weeks of age), and then at 5, 8, 12, and 16 weeks of age. Serum samples were tested using a commercial PRRS ELISA. Both serum and oral-fluid samples were tested for PRRSV by quantitative reverse-transcriptase polymerase chain reaction (PCR), and oral fluids were tested for PCV2 by quantitative PCR. Results: Site One pigs seroconverted to PRRS at 8 to 12 weeks of age, and Site Two and Three pigs at 5 to 8 weeks of age. At all sites, individual serum samples tested PCR-negative for PRRSV in pigs 3 and 5 weeks old, while > 1 sample tested positive in pigs 8, 12, and 16 weeks old. Overall, there was 77% agreement between oral-fluid and serum pen-level results. At all sites, PCV2 was repeatedly detected in oral fluids. Implications: Oral-fluid samples may be used to monitor PRRSV and PCV2 infections in commercial production systems. PRRS virus is detectable in oral fluids for 3 to 8 weeks, and PCV2 may be detectable for > 8 weeks. Sampling at 2- to 4-week intervals is recommended for surveillance of PRRSV and PCV2. | ResumenObjetivos: Validar el uso de fluidos orales para detectar infecciones causadas por el virus del síndrome reproductivo y respiratorio porcino (PRRSV) y el circovirus porcino tipo 2 (PCV2) en tres hatos comerciales. Materiales y métodos: Se recolectaron muestras de suero y fluido oral de un edificio de cada uno de los tres sitios de finalización infectados con el PRRSV. Se tomaron muestras repetidamente de seis corrales por granja (20 a 30 cerdos por corral), iniciando cuando los cerdos entraron a las instalaciones (3 semanas de edad), y después a las 5, 8, 12, y 16 semanas de edad. Se analizaron muestras de suero utilizando un ELISA comercial contra PRRS. Las muestras de suero y fluido oral se analizaron en busca del PRRSV a través de la reacción cuantitativa de transcriptasa reversa en cadena de la polimerasa (PCR), y los fluidos orales se analizaron para PCV2 a través de PCR cuantitativo. Resultados: En el Sitio Uno, los cerdos seroconvirtieron al PRRS entre las 8 y 12 semanas de edad, y en los Sitios Dos y Tres entre las 5 y 8 semanas edad. En todos los sitios, las muestras de suero individuales resultaron PCR negativas al PRRSV en cerdos de 3 a 5 semanas de edad, mientras > 1 muestra resultaron positivas en cerdos de 8, 12, y 16 semanas de edad. Hubo una concordancia del 77% entre los resultados a nivel de corral de suero y fluido oral. En todos los sitios, el PCV2 se detectó repetidamente en fluidos orales. Implicaciones: Las muestras de fluido oral pueden utilizarse para monitorear infecciones de PCV2 y PRRSV en sistemas de producción comercial. El virus del PRRS es detectable en fluidos orales entre 3 y 8 semanas, y el PCV2 puede ser detectado por > 8 semanas. El muestreo a intervalos de 2 y 4 semanas es recomendado para la vigilancia del PRSSV y el PCV2. | ResuméObjectifs: Valider l’utilisation de fluides oraux pour détecter les infections par le virus du syndrome reproducteur et respiratoire porcin (PRRSV) et le circovirus porcin de type 2 (PCV2) dans trois troupeaux porcins commerciaux. Matériels et méthodes: Des échantillons de fluide oral et de sérums ont été prélevés des animaux logés dans un bâtiment sur chacun des trois sites où se trouvaient des animaux en finition infectés par le PRRSV. Six parcs par bâtiment (20 à 30 porcs par parc) ont été échantillonnés de manière répétée, débutant au moment de l’entrée de l’animal dans les facilités (3 semaines d’âge), et par la suite à 5, 8, 12, et 16 semaines d’âge. Les échantillons de sérum ont été éprouvés par ELISA au moyen d’une trousse PRRS commerciale. Les échantillons de sérum et les échantillons de fluide oral ont été testés pour le PRRSV par réaction d’amplification en chaîne par la polymérase (PCR) quantitative utilisant la polymérase réverse et les fluides oraux testés pour PCV2 par PCR quantitative. Résultats: Une séroconversion envers le PRRS a été notée entre 8 à 12 semaines d’âge chez les porcs du site 1, et entre 5 à 8 semaines d’âge pour ceux des sites 2 et 3. À tous les sites, les échantillons individuels de sérum des porcs de 3 à 5 semaines se sont avérés négatifs par PCR pour le PRRSV, alors que > 1 échantillon s’avérait positif chez les porcs âgés de 8, 12, et 16 semaines. De manière globale, il y avait 77% d’accord entre les résultats des fluides oraux et des sérums au niveau des parcs. À tous les sites, le PCV2 était détecté de manière répétée dans les fluides oraux. Implications: Des échantillons de fluide oral peuvent être utilisés pour surveiller les infections par PRRSV et PCV2 dans les systèmes de production commerciale. Le virus du PRRS est détectable dans les fluides oraux pendant 3 à 8 semaines, et le PCV2 peut être détectable pour plus de 8 semaines. Un échantillonnage à des intervalles de 2 à 4 semaines est recommandé pour la surveillance du PRRSV et du PCV2. |
Keywords: swine, oral-fluid
surveillance, polymerase chain reaction, porcine circovirus
type 2, porcine reproductive and respiratory syndrome virus, PCR, PCV, PRRS
Search the AASV web site
for pages with similar keywords.
Received: September
10, 2007
Accepted: October
23, 2007
In both humans and animals, antibodies and pathogens may be detected in oral fluids collected from infected individuals. The presence of antibody in oral fluid was demonstrated as early as 1909.1 Antibody (IgM, IgA, and IgG) is produced locally in salivary glands and lymphoid tissue, but the primary source of antibody in oral fluid is oral mucosal transudate.2 Pathogens in oral fluids may originate in tissues associated with the buccal cavity (eg, classical swine fever virus replicates in the tonsil of the soft palate)3 or reach the buccal cavity from the circulatory system via oral mucosal transudate (eg, hepatitis B virus).4 Examples in which both the agent and antibody are present in oral fluids include foot-and-mouth disease virus in cattle,5,6 Brucella melitensis in humans,7 and feline immunodeficiency virus in cats.8,9
The body of literature on the use of oral fluids in human diagnostics is extensive,10-12 but Archibald et al13 may have been the first to suggest their use as a primary diagnostic specimen. Thereafter, diagnostic assays using oral fluid became available for a variety of infections and infectious agents, including human immunodeficiency viruses,14 measles,15 mumps,16 rubella,17 and hepatitis A, B, and C viruses,18 and others.
In veterinary medicine, oral fluids have been used for detection of Escherichia coli O157:H719,20 and Salmonella in feedlot cattle,19 and diagnosis of feline leukemia virus in cats.21 In swine, specific antibodies were detected in oral fluid following inoculation of pigs with group E Streptococcus,22 Actinobacillus pleuropneumoniae,23 and cholera toxin B subunit.24
Both porcine reproductive and respiratory syndrome virus (PRRSV) and porcine circovirus type 2 (PCV2) have been detected in buccal samples.25,26 Recently, under experimental conditions, oral-fluid samples from pigs inoculated with PRRSV were shown to contain diagnostic levels of virus.27 Here, we report a pilot project validating the use of oral fluids for detection of PRRSV and PCV2 infections in three commercial swine herds.
Materials and methods
Experimental design
Oral-fluid and serum samples were collected on three PRRSV-infected finishing sites stocked with pigs from endemically infected sow farms. Pigs on Site One were sourced from one sow farm, and pigs on Sites Two and Three from a second sow farm. On each site, six pens in one barn (20 to 30 pigs per pen) were sampled repeatedly over time. Samples were collected when the pigs entered the facilities at 3 weeks of age, and then at 5, 8, 12, and 16 weeks of age. At each time point, one oral-fluid sample was collected from each pen, and blood samples were collected from a convenience sample of five pigs per pen. At the end of the collection period, all oral-fluid and serum samples were randomized, relabeled, and tested for PRRSV by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR). In addition, serum samples were tested for anti-PRRSV antibody using a commercial ELISA, and oral fluids were tested for PCV2 by quantitative PCR.
Collection of biological samples
Oral fluids were collected by hanging a length of 5/8-inch cotton rope within the pen for 20 to 30 minutes (Figure 1). At each sampling, the rope was positioned at shoulder height for the pigs in the pen, ie, the length of the rope was adjusted as the pigs grew. Pigs are naturally attracted to the rope and deposit oral fluids during the process of interacting with it (Figure 2).28 After the exposure period, oral fluids were extracted from the rope by wringing the wet end or portion of the rope into a 1-gallon resealable plastic bag (Figures 3 and 4) and clipping a bottom corner of the bag to drain the fluid into a 50-mL centrifuge tube. Samples were stored at -20°C until assayed.
| Figure 1: Equipment used to collect oral fluids
from finisher pigs.
|
| Figure 2: Finisher pigs interacting with a cotton
rope attached to the pen divider.
|
| Figure 3: Collecting the end of a cotton rope containing
oral fluids for analysis.
|
| Figure 4: Oral fluids harvested from the cotton
rope illustrated in Figure 3.
|
Blood samples were collected using a single-use blood collection system (Vacutainer; Becton Dickinson, Franklin Lakes, New Jersey). Blood was centrifuged at 1000g for 10 minutes, and serum was harvested and stored at -20°C.
PRRS virus qRT-PCR
Oral-fluid and serum samples were assayed for PRRSV by qRT-PCR as previously described,28 with minor exceptions. Briefly, viral RNA for qRT-PCR amplification was extracted from 0.14 mL of sample using an Ambion viral RNA kit (Ambion, Valencia, California) according to the protocols recommended by the manufacturer. Real-time RT-PCR quantification was performed using an ABI Prism 7900 HT sequence detection system (Applied Biosystems, Foster City, California). Primers specific for PRRSV open reading frame (ORF) 7 were synthesized by Integrated DNA Technologies, Inc (Coralville, Iowa), and minor groove binder probes were synthesized by Applied Biosystems. The thermal profile for amplification of PRRSV RNA was a reverse transcription at 50°C for 30 minutes, followed by enzyme activation at 95°C for 15 minutes, then 40 cycles of denaturation at 94°C for 15 seconds and a combined annealing-extension step at 60°C for 60 seconds, with fluorescence data capture at the combined annealing-extension stage. For each assay, a standard curve was generated using standards (101 to 106 median tissue culture infectious dose [TCID50] equivalents per mL), and positive and negative control samples were tested with the unknowns. The unit of expression for PRRSV qRT-PCR results was TCID50 equivalents per mL, which represented the quantity of total viral RNA in samples relative to standards in which the amount of infectious PRRSV was quantified using microtitration infectivity assays. A positive sample was defined as a sample that produced a TCID50 estimate in the qRT-PCR assay.
Porcine circovirus type 2 PCR
The presence of PCV2 in oral fluids was assessed by quantitative PCR using a previously described protocol;29 serum samples were not available for testing. Briefly, viral DNA was extracted from 50 μL of each oral-fluid sample using MagMax total viral nucleic acid isolation kit (Ambion, Valencia, California) according to the manufacturer’s instruction. Real-time PCR was performed with TaqMan Fast Universal PCR Master Mix (Applied Biosystems) in 25-μL reaction volumes using 5 μL of extracted template. The PCR primers (Integrated DNA Technologies, Inc) and probe (Applied Biosystems) with 5’ reporter 6-carboxyfluorescein (FAM) and a 3’ TAMRA quencher were designed to detect complementary sequences in ORF1 of PCV2. Primers were added at a final concentration of 20 μM each; the probe was at a final concentration of 25 μM. The PCR amplification was performed on the ABI 7500HT Sequence Detection System (Applied Biosystems). Cycling conditions were as follows: an activation step at 95°C for 20 seconds and then 35 cycles of 3 seconds at 94°C and 30 seconds at 60°C. A set of PCV2 preparations with known virus titer (fluorescent focusforming unit, FFU) was used to generate a standard curve. Samples with a threshold cycle of ≤ 35 cycles were considered positive.
PRRS ELISA
Serum samples were tested for antibodies against PRRSV using the HerdChek PRRS Antibody 2XR Test Kit (Idexx Laboratories, Inc, Westbrook, Maine). Serum samples were assayed according to the manufacturer’s instruction. As recommended by the manufacturer, a positive serum sample was defined as having a sample-to-positive (S:P) ratio ≥ 0.4.
Results
PRRS ELISA
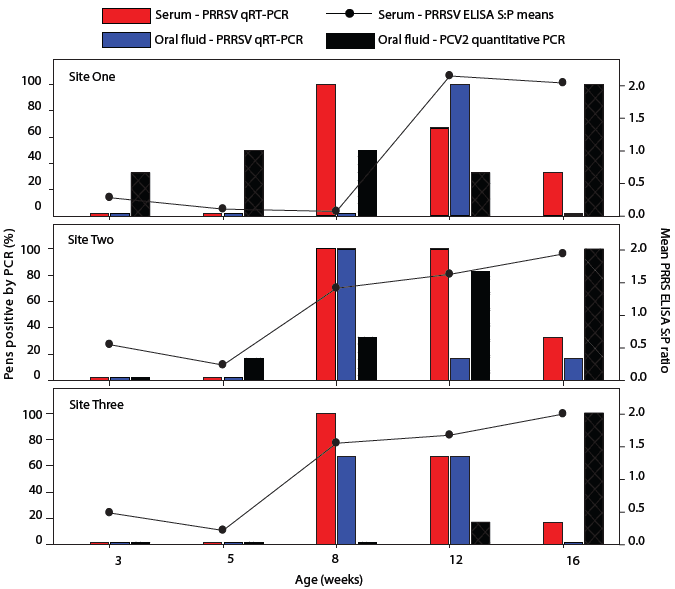
Serum ELISA S:P results are presented as means over time (Figure 5). In pigs at all three sites, S:P ratios declined between placement (3 weeks of age) and 2 weeks post placement. At Sites Two and Three, S:P ratios increased when pigs were 5 to 8 weeks of age. In contrast, pigs at Site One seroconverted between 8 and 12 weeks of age. That is, all Site One pigs (n = 30) were ELISA-negative at 8 weeks of age and ELISA-positive at 12 weeks of age.
PRRS virus qRT-PCR
All serum samples collected when the pigs were 3 and 5 weeks of age tested PCR-negative. At all three sites, one or more serum samples (n = 30 per site at each sampling) tested PCR-positive in pigs 8, 12, and 16 weeks of age. At the pen level, 77% of PRRSV qRT-PCR oral-fluid and serum results were in agreement (Figure 5). PRRS virus was first detected at Sites Two and Three in oral-fluid samples collected at 8 weeks of age, consistent with the serum qRT-PCR results at each site. However, in all 30 pigs 8 weeks of age at Site One, PCR for PRRSV in oral-fluid samples and serum ELISA for PRRSV antibodies were negative, while serum PCR was positive in 25 of 30 pigs sampled at Site One (Figure 5).
| Figure 5: Cumulative results of testing oral fluids
and serum by reverse-transcriptase polymerase chain reaction (RT-PCR) for
porcine reproductive and respiratory syndrome virus (PRRSV), testing oral
fluids for porcine circovirus type 2 (PCV2) by quantitative PCR, and testing
serum by ELISA for antibodies to PRRSV in pigs in three commercial finisher
sites. Pigs entered the facilities at 3 weeks of age. Samples for testing
were collected when pigs were 3, 5, 8, 12, and 16 weeks of age. Blood samples
were collected from a convenience sample of five pigs/pen (total six pens
at each site, 20 to 30 pigs/pen). Pooled oral-fluid samples were collected
by allowing each pen access to a cotton rope for a 20- to 30-minute period.
Results are expressed as the percent of six pens testing positive for the
PCR tests, and the means of sample-to-positive (S:P) ratios for 30 pigs
for the ELISA test, with an S:P ratio of ≥ 0.4 considered positive.
|
Porcine circovirus type 2 PCR
Results of testing oral-fluid samples for PCV2 by quantitative PCR are summarized in Table 1. Two or more oral-fluid samples from Site One tested positive at all sampling points, including all six pens at the last sampling point (16 weeks of age). At Site Two, all pens tested negative at the first sampling, and at Site Three, all pens tested negative at 3, 5, and 8 weeks of age.
Table 1: Results of testing oral fluids for PRRSV by qRT-PCR and for PCV2 by quantitative PCR, and serum samples by ELISA for PRRSV at three commercial finishers endemically infected with PRRSV and PCV2*
* Blood samples collected at placement (3 weeks of age) and at 5, 8, 12, and 16 weeks of age from a convenience sample of five pigs/pen (n = 6 pens, 20 to 30 pigs/pen). Oral-fluid samples were collected on the same days from the same pens by allowing pigs to chew on a cotton rope for a 20- to 30-minute period and collecting the oral fluid from the rope. † Number of positive pens, with pens defined as positive if ≥ 1 sample tested positive. ELISA sample-to-positive ratios ≥ 0.4 were considered positive. PRRSV: porcine reproductive and respiratory syndrome virus; PCV2: porcine circovirus type 2; PCR: polymerase chain reaction; qRT-PCR: quantitative reverse-transcriptase PCR; ELISA: commercial serum ELISA for antibodies to PRRSV. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Discussion
Surveillance, ie, on-going efforts to detect a pathogenic agent or disease, is fundamental to the control, elimination, or eradication of an infectious agent. Current surveillance methods for monitoring PRRSV in the production setting require collection of serum samples from individual animals. The number of samples required, labor, and time associated with serum-based testing are often cost-prohibitive. The most frequent consequence is that surveillance is ineffectively executed or abandoned altogether.
Previous data collected under experimental conditions suggested that PRRSV is detectable in oral-fluid samples for approximately 4 weeks after exposure.28 The objective of this study was to conduct a preliminary assessment of the feasibility of detecting PRRSV in oral-fluid samples collected in an endemically infected commercial population. At pen level, 77% of the PRRSV qRT-PCR oral-fluid and serum results were in agreement. Pen-based oral-fluid sampling offers a simple, nontechnical technique for monitoring PRRSV circulation in a population. Further research under experimental conditions and field settings with matched sera and oral-fluid samples is needed to establish sample size and refine sampling protocols. However, the data reported here and the work previously published28 suggest that a sampling interval of 2 to 4 weeks would be sufficient for timely and effective PRRSV and PCV2 surveillance.
The original experimental design of this study did not include testing for PCV2. Due to the current interest in PCV2, oral-fluid samples were tested for PCV2 by PCR (serum samples were no longer available). Reflecting the ubiquitous distribution of the virus, PCV2 was detected in oral fluids from each of the three sites and, at Site One, two or more PCR-positive oral-fluid samples were recovered at every sampling point. These data suggested that oral-fluid sampling could be used to collect PCV2 for genetic characterization and to monitor circulation of PCV2 in commercial populations.
Implications
- Under the conditions of this study, testing of oral fluids by PCR may be used to detect PRRSV and PCV2 infections in commercial production systems.
- PRRS virus is detectable in oral fluids for 3 to 8 weeks, and PCV2 may be detectable for longer than 8 weeks.
- Sampling at 2- to 4-week intervals is recommended for surveillance of PRRSV and PCV2.
Acknowledgements
The study was supported in part by Pork Checkoff funds distributed through the National Pork Board, Des Moines, Iowa. Photographs are courtesy of Dr Keith Erlandson.
References
1. Pollaci G, Ceraulo S. Das agglutinationsvermögen einiger körperflüssigkeiten beim Mediterranfieber [The agglutinating properties of several body fluids during Malta Fever]. Centralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten (I). Abbott Originale. 1909;52:268–275.
2. Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann N Y Acad Sci. 2007;1098:288–311.
3. Solorzano RF, Thigpen JE, Bedell DM, Schwartz WL. The diagnosis of hog cholera by a fluorescent antibody test. JAVMA. 1966;149:31–34.
4. Ben-Aryeh H, Ur I, Ben-Porath E. The relationship between antigenaemia and excretion of hepatitis B surface antigen in human whole saliva and in gingival crevicular fluid. Arch Oral Biol. 1985;30:97–99.
5. Archetti IL, Amadori M, Donn A, Salt J, Lodetti E. Detection of foot-and-mouth disease virus-infected cattle by assessment of antibody response in oropharyngeal fluids. J Clin Microbiol. 1995;33:79–84.
6. Hyslop NS. Secretion of foot-and-mouth disease virus and antibody in the saliva of infected and immunized cattle. J Comp Pathol. 1965;75:111–117.
7. Wheatcroft MG. A comparative study of human serum and salivary antibody titers in cases of Brucella melitensis infections. J Dent Res. 1957;36:112–117.
8. Poli A, Giannelli C, Pistello M, Zaccaro L, Pieracci D, Bendinelli M, Malvaldi G. Detection of salivary antibodies in cats infected with feline immunodeficiency virus. J Clin Microbiol. 1992;30:2038–2041.
9. Yamamoto JK, Sparger E, Ho EW, Andersen PR, O’Connor TP, Mandell CP, Lowenstine L, Munn R, Pedersen NC. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988;49:1246–1258.
10. Kaufman E, Lamster IB. The diagnostic applications of saliva - a review. Crit Rev Oral Biol Med. 2002;13:197–212.
11. Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19:119–125.
12. Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76.
13. Archibald DW, Zon LI, Groopman JE, Allan JS, McLane MF, Essex ME. Salivary antibodies as a means of detecting human T cell lymphotropic virus type III/lymphadenopathy-associated virus infection. J Clin Microbiol. 1986;24:873–875.
*14. Nightingale SL. From the Food and Drug Administration. JAMA. 1995;273:613.
15. Helfand RF, Kebede S, Alexander JP, Alemu W, Heath JL, Gary HE Jr, Anderson LJ, Beyene H, Bellini WJ. Comparative detection of measles-specific IgM in oral fluid and serum from children by an antibody-capture IgM EIA. J Infect Dis. 1996;173:1470–1474.
16. Warrener L, Samuel D. Evaluation of a commercial assay for the detection of mumps specific IgM antibodies in oral fluid and serum specimens. J Clin Virol. 2006;35:130–134.
17. Vijaylakshmi P, Muthukkaruppan V, Rajasundari A, Korukluoglu G, Nigatu W, Warrener LA, Samuel D, Brown DW. Evaluation of a commercial rubella IgM assay for use on oral fluid samples for diagnosis and surveillance of congenital rubella syndrome and postnatal rubella. J Clin Virol. 2006;37:265–268.
18. Amado LA, Villar LM, de Paula VS, de Almeida AJ, Gaspar AMC. Detection of hepatitis A, B, and C virus-specific antibodies using oral fluid for epidemiological studies. Memorias do Instituto Oswaldo Cruz. 2006;101:149–155.
19. Smith DR, Moxley RA, Clowser SL, Folmer JD, Hinkley S, Erickson GE, Klopfenstein TJ. Use of rope devices to describe and explain the feedlot ecology of Salmonella by time and place. Foodborne Pathol Dis. 2005;2:61–69.
20. Stanford K, Bach SJ, Marx TH, Jones S, Hansen JR, Wallins GL, Zahiroddini H, McAllister TA. Monitoring Escherichia coli O157:H7 in inoculated and naturally colonized feedlot cattle and their environment. J Food Prot. 2005;68:26–33.
21. Lewis MG, Wright KA, Lafrado LJ, Shanker PJ, Palumbo NE, Lemoine ED, Olsen RG. Saliva as a source of feline leukemia virus antigen for diagnosis of disease. J Clin Microbiol. 1987;25:1320–1322.
22. Isaki L, Bairey M, van Patten L. Response of vaccinated swine to group E Streptococcus exposure. Cornell Vet. 1973;63:579–588.
23. Loftager MK, Eriksen L, Nielsen R. Antibodies against Actinobacillus pleuropneumoniae serotype 2 in mucosal secretions and sera of infected pigs as demonstrated by an enzyme-linked immunosorbent assay. Res Vet Sci. 1993;54:57–62.
24. Hyland K, Foss DL, Johnson CR, Murtaugh MP. Oral immunization induces local and distant mucosal immunity in swine. Vet Immunol Immunopathol. 2004;102:329–338.
25. Allan GM, Ellis JA. Porcine circoviruses: A review. J Vet Diagn Invest. 2000;12:3–14.
26. Wills RW, Zimmerman JJ, Yoon KJ, Swenson SL, Hoffman LJ, McGinley MJ, Hill HT, Platt KB. Porcine reproductive and respiratory syndrome virus: routes of excretion. Vet Microbiol. 1997;57:69–81.
27. Prickett JR, Simer R, Christopher-Hennings J, Yoon K-J, Evans RB, Zimmerman JJ. Detection of porcine reproductive and respiratory syndrome virus infection using pen-based oral fluid samples: A longitudinal study under experimental conditions. J Vet Diagn Invest. In press.
28. Opriessnig T, McKeown NE, Harmon KL, Meng XJ, Halbur PG. Porcine circovirus type 2 infection decreases the efficacy of a modified live porcine reproductive and respiratory syndrome virus vaccine. Clin Vaccine Immunol. 2006;13:923–929.
29. Opriessnig T, Yu S, Gallup JM, Evans RB, Fenaux M, Pallares F, Thacker EL, Brockus CW, Ackermann MR, Thomas P, Meng XJ, Halbur PG. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Vet Pathol. 2003;40:521–529.
*Non-refereed reference.