Sodium ion toxicosis (salt toxicity or salt poisoning) is a condition that occurs when animals are without water for a time and then consume high volumes of water in a short period. Although rare, intoxication through the consumption of excessive concentrations of sodium within feed can occur. Excess sodium intake during a period of water deprivation may exacerbate and expedite complications. Ingestion of brackish or salt water may also result in sodium ion intoxication. Following an extended period without water, sodium increases within the brain through passive diffusion. As a result, glycolysis is inhibited and the production of adenosine triphosphate (ATP), a source of energy, is halted. With ATP production diminished, sodium-potassium dependent ATPase pumps, can no longer actively transport sodium out of the neural tissue.1 Clinical complications occur when unrestricted access to water is provided. The abrupt and rapid increase of water consumption results in a sudden osmotic shift within the brain by the influx of water leading to swelling of the brain, neurologic deficits, and eventual death. Returning animals to normal levels of water consumption to prevent sodium ion intoxication requires time and gradual re-introduction to water.2 This practice tip provides procedures for re-introducing animals experiencing sodium ion toxicosis to water in a controlled manner.
Clinical signs
Initial signs of sodium ion intoxication due to water deprivation may include thirst, anorexia, and constipation characterized by firm feces, and then usually followed by central nervous system signs. Severe cases of sodium ion intoxication result from extended periods without water (> 24 hours) and are exacerbated upon rapid rehydration. The amount of time that animals are deprived of water resulting in sodium ion intoxication is dependent on the environment and may be less than 24 hours. Severe cases often result in pigs exhibiting intermittent convulsive seizures with opisthotonos, often starting from a “dog-sitting” position.1-3 As they are centrally unaware, affected animals may appear to wander aimlessly, exhibit head pressing, head jerking, jaw chomping, and appear to be blind and deaf. Moribund pigs become comatose, often lying on their sides with continuous paddling. Most moribund animals will die within a few hours and up to 48 hours after they begin displaying these clinical signs.
Diagnosis
Diagnosis is initially established based on clinical signs coupled with a history of prolonged water deprivation, excessive sodium intake, or both. Confirmation of the initial diagnosis is recommended and can be confirmed by submitting fresh and formalin-fixed brain tissue from affected animals to a veterinary diagnostic laboratory.1,2,4 In addition, a thorough history should be collected to rule out other potential agents, such as organophosphate and carbamate pesticides, that may cause similar clinical symptoms.4 An assessment of how long pigs may have been deprived of water or exposed to excessive concentrations of sodium should be performed. Facilities, equipment, and water flow and availability should also be evaluated.1
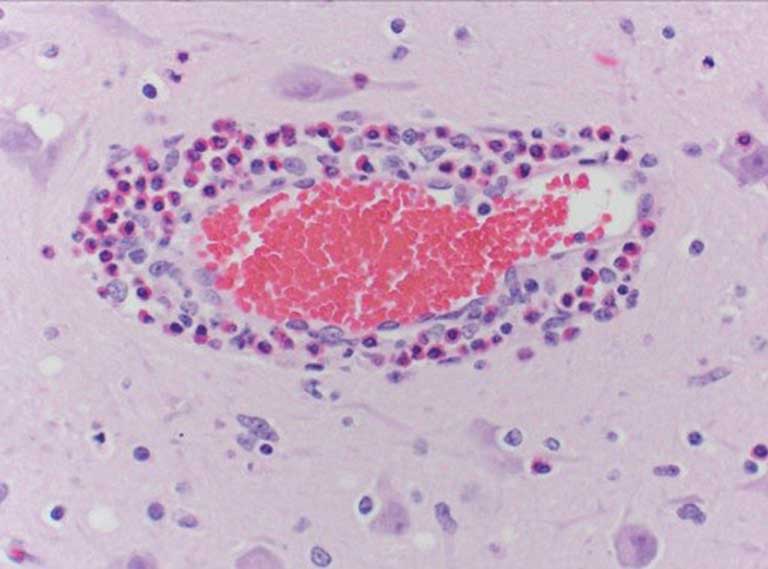
Histologic examination of the cerebrum often shows a pathognomonic eosinophilic meningoencephalitis characterized by cuffing of meningeal and cerebral vessels (Figure 1) with eosinophils in acutely affected pigs (< 12 hours from unrestricted access to water following deprivation). In addition to the eosinophilic perivascular cuffing, necrosis of neurons and laminar cortical necrosis, also known as polioencephalomalacia, may be observed.1,2 A definitive diagnosis of sodium ion intoxication should not be solely dependent on formalin-fixed brain and presence of eosinophilic perivascular cuffing. Animals that have been treated, have started to recover, or sampled 24 hours following initial complications are likely to exhibit few eosinophils as they are typically replaced by macrophages. When examining fixed tissue microscopically, only a single cell layer can be observed. There is a possibility that the aforementioned pathognomonic lesions may not be observed. Therefore, multiple sections should be submitted for evaluation.2 Analysis of brain sodium concentrations is an additional method for diagnosing sodium ion intoxication due to water deprivation. A brain sodium concentration greater than 1800 ppm in fresh brain (wet) is an indicator of salt toxicity.1 In such situations, it is not uncommon that brain sodium concentrations exceed 2000 ppm.2 In treated or recovering individuals, brain sodium concentrations may be normal (< 1800 ppm).3 Analysis of brain sodium can only be performed on fresh tissue as buffered and nonbuffered formalin can falsely elevate or lower brain sodium, respectively.5 Serum, ocular fluid, and cerebral spinal fluid (CSF) can also be used to evaluate sodium concentrations. Other than a spinal tap, serum serves as the only ante-mortem sample to aid in diagnosing dehydration and sodium ion intoxication. Evaluation of serum at a clinic may be performed to offer evidence of dehydration and hypernatremia in living affected animals. Sodium within serum and CSF > 160 mEq/L are supportive of a diagnosis. The cleanliness of a CSF sample must be taken into consideration as contamination by blood and other material may occur during the collection process.2
Causes of dehydration
Dehydration is an event in which an organism releases more water than it consumes. Water release from an organism can be in forms of excretions (urinary, fecal, and sweat) and breath. Water deprivation can occur at various times throughout an animal’s life. For example, disease can cause a fever which reduces the animal’s intake of water. Facility issues such as broken, frozen, or clogged water lines or medicators can also create periods of water absence. Long transportation times and producer error when switching water lines during times of mass water medication can result in pigs going without water for an extended time. High air temperatures in which the body must compensate for temperature regulation can result in significant water loss. For example, the deprivation time would be shorter in animals during hot summer months than those in cooler environments. Animals may have water available to them at all times, but that does not mean that they drink it. In situations where water has always been available, an evaluation of water meters to monitor usage should be performed. Although water may have been available, there are circumstances in which water consumption may have been stagnant or decreased. These include unpalatable water due to either additives such as medications or water sourced from a new well in which a salt vein was struck and no quality testing was performed. The height of the waterers and familiarity of the watering system to animals should also be considered. Animals not familiar with new sources of water, ie, cup vs nipple waterer, may not approach or use the waterer.1-4
Interventions
More severe dehydration results in a longer period of rehydration. Any supplementary water interventions should be continued until the pigs quit fighting for water or lose interest in the water source. When serum sodium levels cannot be actively monitored, individuals should assume that pigs should be rehydrated at a rate of 0.5% of body weight each hour.3 However, when serum sodium levels can be measured, the goal would be to replace 50% of the free water deficit (FWD) within the first 24 hours.2 The remaining deficit should be replaced within the following 48 hours.3 Liters of FWD is calculated as FWD = 0.6 × BW × (current Na+ )/desired Na+ – 1) where BW is pig body weight in kilograms and Na+ is serum sodium concentration in milliequivalents per liter.1-3
When reintroducing water, methods need to be conducted over multiple hours until the pigs lose interest in the water source to successfully reduce the risk of sodium toxicosis. An anti-inflammatory protocol based on veterinarian input should be included as an intervention for pigs that are severely impacted. Pigs should never be given electrolytes in the water during a rehydration event.
To generate a list of interventions used in the field, members of the American Association of Swine Veterinarians (AASV) were asked via the AASV-L Email Discussion List6 how they have brought water deprived pigs back onto water. The following is a compilation of suggestions from veterinary practitioners (N = 15) for the various methods they have used to minimize the clinical signs of salt toxicity while slowly re-introducing the pigs to water. The methods listed are not all inclusive and, in some scenarios, the producer may choose to use multiple methods of rehydration based upon pen stocking density and access to the water supply.
Turning the water on and off
Water can be turned on and off for a variety of timeframes. Some of the suggested water intake patterns were 1) on for 5 to 10 minutes within each 30 minute period; 2) on for 3 to 5 minutes and off for 5 to 10 minutes; 3) 15 minutes on and 2 hours off; and 4) fill water cups and repeat in 10-minute increments. If the stocking density within the pen is high or the number of functional drinkers available is reduced, it is possible that the most aggressive pigs in the pen will get more than their share of the available water in the limited time the water is turned on. Less aggressive pigs may drink the spillage from other pigs. In this situation, an additional water supply may be needed to increase the odds that rehydration starts in the greatest percentage of pigs possible.
Use of floor space
When a large number of pigs are affected, as an adjunct to turning on and off water as previously described, producers can use the floor to create water access. Methods to employ could include 1) running water on slatted or partially slatted floors by using either a garden hose or buckets; 2) placing snow on the floors or slats; and 3) removing feed from feeders, pouring in small amounts of water, and periodically refilling the water once the feeders have been emptied.
Misters
If functional spray misting or drip cooling systems are available in the facility, they can be used to slowly rehydrate the pigs. It should be kept in mind that when misters are used, feed could get wet and so covers over the feeders should be considered. Misters can be run at intervals of 15 minutes on and then 15 minutes off.
Creep feeders
Creep feeders or capped PVC tubing cut in half to create troughs can be placed into pens to quickly allow water access to a large group of pigs. Small amounts of water would need to be added over time.
Veterinary intervention
A veterinarian, veterinary technician, or a trained caretaker under direct veterinary supervision may employ other techniques to restore fluid, such as rectum infusion or intraperitoneal injection.
Acknowledgments
The authors would like to thank the swine veterinary community that responded via the AASV listserv for their suggestions and practice tips for this publication.
Conflict of interest
None reported
Disclaimer
Scientific manuscripts published in the Journal of Swine Health and Production are peer reviewed. However, information on medications, feed, and management techniques may be specific to the research or commercial situation presented in the manuscript. It is the responsibility of the reader to use information responsibly and in accordance with the rules and regulations governing research or the practice of veterinary medicine in their country or region.
References
1. Niles G. Metals and Minerals: Sodium. In: Plumlee KH. Clinical Veterinary Toxicology. Mosby; 2004:218-221.
2. Osweiler GD. Water Related Toxicoses: In: Osweiler GD. Toxicology. Wiley-Blackwell; 1996:355-357.
3. Ensley SM, Radke SL. Toxic Minerals, Chemicals, Plants, and Gases. In: Zimmerman J, Karriker L, Ramirez A, Schwartz K, Stevenson G. Diseases of Swine. 11th Ed. Wiley-Blackwell; 2019:1082-1083. https://doi.org/10.1002/9781119350927.ch70
4. Thompson LJ. Salt toxicosis in animals. In: Merck Manual Veterinary Manual. Revised May 2022. Modified November 2022. Accessed April 9, 2023. https://www.merckvetmanual.com/toxicology/salt-toxicosis/salt-toxicosis-in-animals
*5. Schrunk D, Ensley S, Radke S, Magstadt D. The effects of formalin fixing on trace mineral status. In: Proceedings of the AAVLD Annual Meeting. American Association of Veterinary Laboratory Diagnosticians. 2017. San Diego, CA.
*6. Recovery from Water Deprivation. AASV-L Email Discussion List. October 19, 2022.
* Non-refereed references.
