Diagnostic Notes
Diagnostic Approaches to Swine Central Nervous System Disorders - A Practitioner's Perspective
Chris J. Rademacher, DVM
Rademacher CJ. Diagnostic Approaches to Swine Central Nervous System Disorders. J Swine Health Prod. 2001;9(1):31-33. Also available as a PDF (112k).
New Fashion Pork, Inc. Jackson, Minnesota 56143
Editor's Note: This article is the third in a series describing practitioner's diagnostic approaches to common swine disease syndromes. --David H. Zeman - Diagnostic Notes Editor
Diseases of the central nervous system (CNS) in swine are commonly seen in our practice and are usually caused by infectious disease. However, CNS disease may be genetic, congenital, or toxic in origin. Usually, neurological disorders are manifest by signs such as ataxia, incoordination, abnormal gait, paresis, paralysis, tremors, paddling, opisthotonos, convulsions, nystagmus, and death.
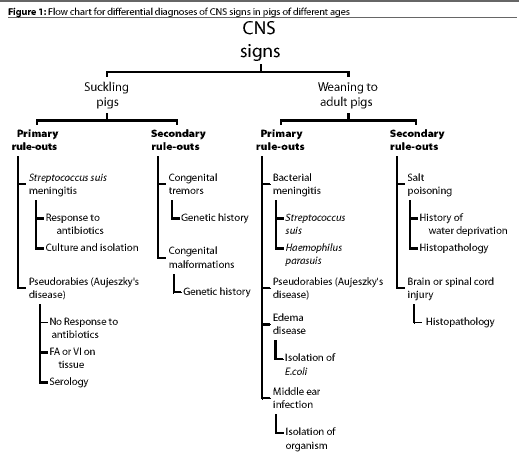
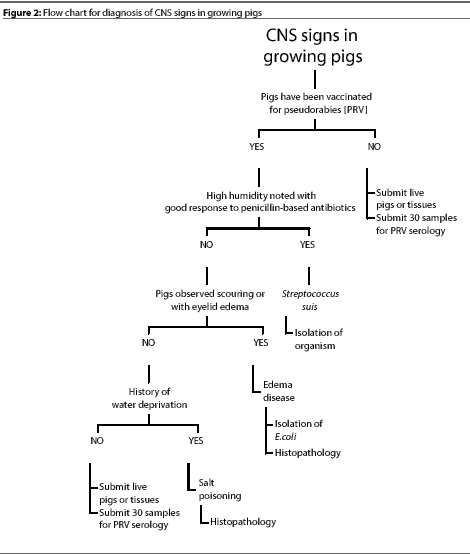
Figure 1 shows a flow chart that we use for differential diagnose
of CNS disease in pigs of different ages. The primary rule-outs
are diseases that occur most often and should be considered first
when CNS signs are observed. If the primary etiologic agents are
ruled out, the secondary rule-outs should be considered. Figure
2 shows a flow chart for diagnosis of CNS disease in pigs from
weaning to maturity. While the list of CNS diseases included in
these charts is by no means comprehensive, it is a good starting
point for production personnel.
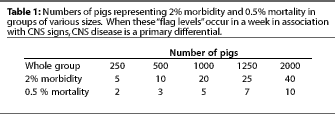
Our production staff alert a staff veterinarian if the number
of pigs with CNS signs (morbidity) during a given week exceeds
a "flag level" of 2%, or the number of acute deaths
(mortality) exceeds 0.5% (5 pigs out of 1000). Table 1 shows how
these "flag levels" relate to the number of affected
pigs in various group sizes.
When visiting a facility reporting CNS signs, I first look at the daily production reports and visit with the site manager to get a history. The information that I'm looking for is
- Length of time pigs have been displaying clinical signs
- Age of pigs when clinical signs were first noted
- Number of pig deaths
- Number of pigs displaying clinical signs
- Number of pigs individually treated and what they were treated with
- Response to injectable antibiotics
- Daily water meter readings
- High and low thermometer readings
- Relative humidity readings
- Ventilation control settings
This information usually helps to narrow down the list of possible diagnoses. If early- to mid-nursery phase pigs show recumbency, paddling, opisthotonos, nystagmus, or acute death, but respond well to injectable penicillin, we usually assume we are dealing with Streptococcus suis meningitis. Decreased water consumption may indicate problems with water availability, or may be a sign that pigs are not feeling well. A 20 to 30% decrease in water consumption may be observed in pigs with an acute viral infection, such as swine influenza or Pseudorabies, one or two days before gross clinical signs appear. High and low thermometer and relative humidity readings may reveal that temperature extremes or improper ventilation rates are responsible for stressing the pigs and causing clinical signs.
The next step in diagnosis is to do a walk-through of the facility with the staff. I walk down the alleyway, looking at a few individual pigs within each pen, to gauge the attitude of the entire group. This is helpful in estimating the prevalence of clinical signs. Does the entire group look stressed, with some pigs demonstrating gross neurologic signs, or are just a few random pigs affected? Are the pigs with clinical signs in just one or two pens, or are they scattered throughout the barn?
Finally, I examine the pigs showing clinical signs, usually by moving an affected animal out of the pen and into the alley, in order to determine exactly which neurological signs the pig is displaying. A pig with an inner ear infection may demonstrate a head tilt and circling behavior that may not be evident when it is in a pen with 25 to 30 other pigs.
I usually sample two to three pigs per group for a CNS diagnostic workup. I prefer to select one or two live, untreated pigs showing clinical signs, and if possible, have the live pigs delivered to the diagnostic lab. This allows the pathologist to select the appropriate tissues and eliminates the possibility of tissue degradation during shipment. If it is not possible to deliver live pigs to the lab, I euthanize them and perform a necropsy.
I usually take fresh and formalin fixed tissue samples of brain, tonsil, liver, spleen, lungs, enlarged regional lymph nodes, heart, and other tissues that appear abnormal. For example, if the intestines appear to be inflamed or contain excessive amounts of fluid, fresh samples of intestinal tissue are taken to confirm, or rule out, edema disease.
Serology may be useful, particularly for Pseudorabies virus (Aujeszky's disease), as infected pigs may have seroconverted by the time they show clinical signs. Samples for serology should be taken both from pigs showing clinical signs and from some asymptomatic animals. I usually take 10 samples per affected group. These animals are tagged, and convalescent serum is taken 2 to 3 weeks later.
Case report
A finishing site experienced no mortality for 8 days after stocking with 1250 10-week-old feeder pigs. Acute deaths occurredduring the next 2 days, 11 the first day and 14 the second, with pigs demonstrating various types of CNS signs. Affected pigs did not respond to treatment with penicillin.
The pigs had been vaccinated for Pseudorabies with an approved, MLV, gene-deleted vaccine 8 days previous to the first deaths. Daily water consumption and environment controller settings were normal. The environment appeared very humid and the flooring was very wet. The pigs were huddling and piling in the center of the pens, and profuse, watery diarrhea was noted in 50% of them. A variety of CNS signs, ranging from front-leg paralysis to staggering, uncoordinated movements, head tilting, and severe ataxia, were observed in approximately 2 to 3% of the pigs (25 to 40 animals). Eyelid edema was prominent in all pigs showing CNS signs.
As the facility was located within 30 miles of the state diagnostic lab, one pig that had died acutely, and two live pigs displaying CNS signs, were immediately submitted for diagnosis. Necropsy revealed fluid-filled small intestines and dilated colons. One pig had severe gastric mural edema. Histopathology showed mild, nonsuppurative enteritis and edema of the lamina propria, with large numbers of coliforms colonizing the enterocyte villi. Severe submucosal edema was noted in the stomach and the colon. There was hypertrophy of capillary endothelium and a focus of malacia in the brainstem.
A hemolytic strain of E.coli, sensitive to apramycin, gentamicin, ceftiofur, and trimethoprim-sulfamethoxazole, was isolated from the small intestines. PCR testing on this isolate revealed an F18 strain of E.coli carrying the SLT-IIe gene that produces verotoxin. When the group was treated with water-soluble apramycin, acute mortality stopped almost immediately. Pigs with eyelid edema or diarrhea, without CNS signs, responded well to injectionsof ceftiofur, but pigs with CNS signs did not respond to treatment.
