Original research
In-herd prevalence of Salmonella in 25 selected Minnesota swine farms
Allan R Carlson, DVM; Thomas Blaha, DVM, PhD.
Carlson AR, Blaha T. In-herd prevalence of Salmonella in 25 selected Minnesota swine farms. J Swine Health Prod. 2001;9(1):7-10. Also available as a PDF (132k).
AC: Swine Health Center, 7295 Blackwell Drive Southwest, Farwell, MN 56327, phone: (320)886-3021, email: swinehealthcenter@hotmail.com; TB: Department of Animal Science/Veterinary Medicine, University of Minnesota, 1988 Fitch Ave., St. Paul, 55108, phone (612)625-8290, fax: (612)625-1210, email: blaha002@tc.umn.edu. Address all correspondance to Dr. Carlson.
Summary
Objective: To assess in-herd prevalence of Salmonella and determine the serovar patterns in a sufficient number of swine herds for a long-term, multi-phase study on the infection-contamination-infection cycle of Salmonella.
Materials and methods: 25 Minnesota swine herds were selected for the study. None of the participating farms identified clinical Salmonella as a problem in their operations. Ileocecal lymph nodes of swine from the 25 farms were collected repeatedly at slaughter by bluntly dissecting lymph nodes from the caudal mesentery. These nodes were macerated and cultured for Salmonella.
Results: One or more animals from 16 of 25 farms were Salmonella-positive on the basis of ileocecal lymph node culture at slaughter. The herd prevalence rate was 64%. Overall, 3.69% of animals were Salmonella-positive at slaughter. The percentage of Salmonella-positive animals in each shipment varied from 0% to 33.3%. The total number of different Salmonella serovars isolated from any of the farms varied from one to nine. All Salmonella serovars isolated were non-species adapted, including serovars Agona, Infantis, and Newhaw. Serovar Choleraesuis was not identified.
Implications: Zoonotic Salmonella serovars, which cause only clinically inapparentinfections in pigs, are prevalent in swine herds, and are of concern to the food industry. The pattern of Salmonella serovars on a farm tends to be specific but variable. Prevalence of Salmonella varies greatly not only among farms, but also within farms, from one production site to another, and from shipment to shipment.
Keywords : swine, Salmonella,
herd prevalence, in-herd prevalence, sampling strategy
: swine, Salmonella,
herd prevalence, in-herd prevalence, sampling strategy
Received: May 1, 2000
Accepted: July 25, 2000
The World Health Organization (WHO) considers food-bornedisease, which is most commonly caused by bacteria,1,2 to be one of the most widespread health problems in the world. Salmonella is the most discussed and studied of the food-borne bacterial illnesses. Pathogenic bacteria, including Salmonella, can be found in fresh meat and other consumable goods, and may be transmitted to consumers and occupationally exposed persons.2
Until recently, food-borne salmonellosis in man has been associated primarily with poultry and eggs; however, documented outbreaks have been traced back to pork. The risk of human disease due to Salmonella in pork products is real, and needs to be addressed throughout the animal production chain.3,4
In 1996, the United States Food Safety Inspection Service (FSIS) finalized legislation that attempts to reduce contamination of meat products with food-borne pathogens and to increase food safety in the United States. The Hazard Analysis/Critical Control Point Pathogen Reduction Act was published in June 1996, and the program was introduced in major slaughtering plants in the United States in January 1998.5 The act also established new targets for pathogen reduction, based on standardized sampling during production as well as of the final product. Salmonella is one of the indicator pathogens being monitored at slaughtering facilities.
Infected animals are believed to be the principle source of non-typhoid Salmonella serovars that infect humans through meat consumption. Carrier animals are the major source of contamination for other animals, both directly, and indirectly via the environment during transport and at the slaughter facility. Contaminated animals contact equipment and personnel in the facility, increasing the risk that the final product will be contaminated with Salmonella. Reducing the number of infected animals entering the slaughter facility is one means of reducing risk of Salmonella contamination of meat products.
Most studies on Salmonella infections at farm level have focused on animal-to- animal transmission. Fecal-oral transmission has been well documented, and is the primary mode of Salmonella dissemination within a population.6 However, detailed investigations have not been completed identifying the specific sources of Salmonella when a swine population is first infected, nor when the infection is perpetuated on the farm. Information on the dynamic nature of the Salmonella infection-contamination-infection cycle in swine herds is a prerequisite for effective control measures of Salmonella at the farm level.
Kasbohrer and Blaha7 reported that environmental sources introduced Salmonella into European poultry flocks that had been established with Salmonella-free chicks. Prevalence of Salmonella in chickens at slaughter was almost as high in flocks that had started with Salmonella-free day-old chicks as in flocks that had started with Salmonella-positive day-old chicks. This research clearly demonstrated that the environment was the source of infection for flocks subsequently housed on the same premise. Satisfactory control was obtained only with a drastic reduction of environmental Salmonella, achieved by targeted cleaning and disinfection before introducing Salmonella-free chicks.7
Similar results have been reported for swine.8 Salmonella was eliminated from herds by use of segregated early weaning technologies. Piglets were weaned at an early age, reared in a research isolation facility, and then divided into two groups. One group remained in isolation, while the other was moved to a traditional finishing facility. The animals that remained in isolation remained Salmonella-free, while the others became positive, demonstrating that animals are not necessarily the source of Salmonella infection.
Research into swine production management practices will clarify our understanding of the dynamics of Salmonella in herds and on farms. It may be possible to reduce the overall prevalence of Salmonella on farms by controlling, reducing, or possibly eliminating specific sources of Salmonella.
This paper reports on the first phase of a University of Minnesota long-term study on the sources and maintenance of Salmonella infections on swine farms. As the first step, the prevalence of Salmonella in selected Minnesota swine herds was determined by identifying Salmonella serovars isolated from slaughter hogs.
Materials and methods
As the multi-phase study required long-term participation, producers were non-randomly selected on the basis of the investigator's knowledge of the farm's production system, and the proximity of the farm to the principle investigator and to the selected slaughtering facility. Producers were interviewed to determine their willingnessto cooperate in a long-term study. Twenty-five Minnesota swine producers agreed to take part in the study and have their animals slaughtered at one facility for consistency and ease of sample collection. Market-ready, 94.5-kg to 135-kg (210- to 300-lb) animals could be slaughtered several days of each week during the study, allowing producers to move animals according to their own production schedules.
Animals were identified by a farm-specific tattoo before transportation to the slaughter plant. Source farms were then assigned a number that would identify all samples from that farm throughout the study.
Ileocecal lymph nodes were harvested by blunt dissection to prevent contamination by intestinal content. The ileocecal lymph node was selected for culture because it is the tissue most consistently colonized with Salmonella in an infected animal,9 and because this node is easy to harvest from the mesenteric viscera without contaminating either the sample or the carcass.
Farms were repeatedly sampled over an 8-month period, and, depending upon farm size and slaughter animal marketing frequency, 10 to 70 lymph nodes were sampled on two to 20 occasions per herd. Harvested lymph nodes were packaged separately for each farm and transported to the laboratory on cold packs.
Ileocecal lymph nodes were cultured within 24 hours of collection. The node surface was decontaminated by flaming with 95% alcohol, then the node was placed in a sterile sample bag and macerated with a hammer to expose the interior. Depending on the size of the node, samples were incubated in 20 to 100 mL of freshly prepared tetrathionate broth (Tetrathionate Broth Base, Difco Laboratories, Detroit, Michigan) containing iodide (potassium iodide, certified A.C.S., Fisher Scientific, Fair Lawn, New Jersey). Cultures were incubated in sterile sampling bags or in sterile 25x150 mm disposable tubes for 22 to 26 hours at 37 degrees C.
After the initial incubation of each bag or tube, a swab was used to transfer 100 µL of the tetrathionate broth to 10 mL of Rappaport-Vassiliadis R10 Broth (Difco Laboratories) in 16x150 mm sterile disposable tubes, which were then incubated at 37 degrees C for another 22 to 26 hours.
A 10-µL loop (Nagle Nune International, Denmark) was used to streak the Rappaport-Vassiliadis broth onto XLT-4 Agar (Difco Laboratories) and BG Sulfur Agar (Difco Laboratories). Plates were incubated for 22 to 26 hours at 37 degrees C.
Single colonies that appeared to be Salmonella were transferred into tubes of Triple Sugar Iron Agar; Lysine Iron Agar; Motility, Indole and Ornithine Decarboxylase (MIO); and Urea Agar (Difco Laboratories). Tubes were incubated for 22 to 26 hours at 37 degrees C. Colonies confirmed to be Salmonella on the basis of the biochemical test results were serotyped using Salmonella O-and H-antigen polyvalent, group, and monovalent sera (Difco Laboratories).
Statistical analysis
A chi square test was used to determine whether Salmonella culture prevalence differed among farms, using Statistix for Windows version 1.0.
Results
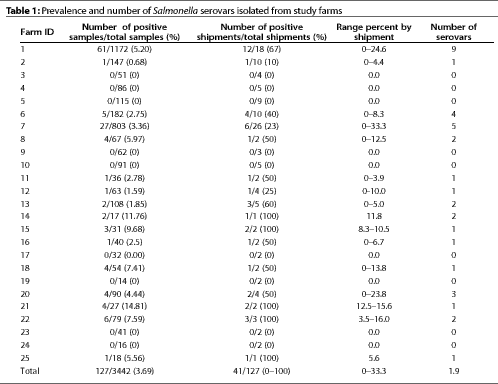
Salmonella serovars were isolated from 127 of 3442 lymph nodes cultured, resulting in an animal prevalence of 3.69%. Lymph nodes from one or more animals on 16 of the 25 participating farms were positive for Salmonella, resulting in a farm prevalence of 64%. Salmonella culture prevalence differed among farms (P<0.05). Statistical tests were not done to determine differences between individual pairs of farms.
One or more animals in 41 of 127 shipments were positive for Salmonella, resulting in a shipment prevalence of 32.3%. Salmonella-positive animals were present in 10% to 100% of shipments from Salmonella-positive farms, and in 0% to 33.3% of shipments overall.
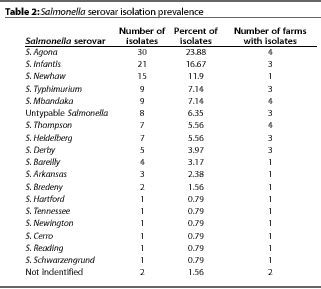
Eighteen different Salmonella serovars were isolated
from the 127 positive animals, with each positive farm having
one to nine serovars (Table 1). The most common serovars were
Agona, Infantis, Newhaw, Typhimurium, and Mbandaka (Table 2).
From several farms, we isolated certain serovars that were
unique to that farm (Table 3). In some cases, the unique serovar
was isolated across several separate shipments from the same farm.
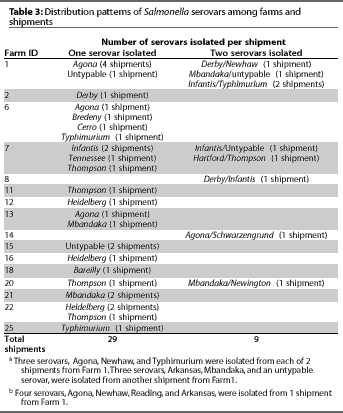
From several farms, more than one serovar was isolated in a single shipment. For farms from which we isolated multiple serovars, the serovars we isolated differed from shipment to shipment (Table 3).
Discussion
Isolation and typing of Salmonella serovars was intended to determine the prevalence of Salmonella on the selected farms, as well as to provide isolates for further investigations. Prevalence of Salmonella in slaughter swine has been investigated in the United States and abroad. In this study, only selected herds were sampled, and the 3.7% animal prevalence we found is not representative of the Salmonella prevalence in Minnesota swine. However, it is similar to that found in swine herds in the USA (6.2%),9 Canada (5.2%),10 and Germany (3.3%).11
The herd prevalence of 64%, measured by repeated sampling, is higher than the prevalence reported in cross-sectional studies in the USA (47% and 38.2%),1,9 Canada (26.2%),10 and Germany (24%).11 If only one shipment had been sampled, most of the participating farms in this study would have been found negative. Moreover, if only the first shipment sampled from each farm had been used to make the determination, the results would have been comparable to those in the cross-sectional studies.
There was a wide variation in the prevalence of Salmonella in slaughter animals in separate shipments from the same farm, and even within groups of finishing animals. This suggests that Salmonella infection is dynamic both in a herd and within groups in the herd. Therefore, repeated sampling of shipments or groups would more accurately assess the herd status of Salmonella than sampling a larger number of animals on a single occasion.
Several farms in this study marketed slaughter animals from separate production sites. In two cases where animals were from environmentally separate sites, one shipment was negative and the other positive for Salmonella. This suggests that all environmentally independent production sites must be sampled repeatedly to accurately assess the level and prevalence of Salmonella in the farm production system.
In this study, animals in a shipment were usually infected with a single Salmonella serovar. However, multiple serovars were isolated from shipments from some farms, and the same farms tended to have shipments infected with multiple serovars (Table 3).
Implications
- Multiple pigs and multiple sampling time periods are needed to accurately assess the Salmonella status of a farm system or even a particular group of animals.
- Although farms and production groups tend to have a specific pattern of Salmonella serovars, this pattern does change, sometimes over relatively short periods of time, suggesting that new serovars are introduced to the animals from their environment.
- Contamination of meat products with Salmonella could be reduced if high prevalence herds were separated from low and medium prevalence herds at slaughter, decreasing cross-contaminationduring the slaughter procedure.
- Identification of high prevalence herds provides an opportunity to reduce the Salmonella load on these farms, and subsequently, the number of high prevalence hogs in slaughter facilities.
References--refereed
4. Blaha T. Preharvest food safety and slaughter perspectives. Rev Sci Tech. 1997;16:489-495. Review.
5. United States Department of Agriculture (1996): USDA-Food Safety and Inspection Service. Final Rule on Pathogen and HAACP systems. 9 CFR part 304. Fed Register, July 25, 1996, 61:38806-38989.
6. Wilcock BP. Salmonellosis. In: Leman AD, ed. Diseases of Swine. 6th edition. Ames, Iowa: Iowa State University Press. 1986:508-520.
References--non-refereed
1. Stoehr K, Meslin FX. Foodborne salmonellosis: Impact on public health and economics. Proc AD Leman Conference. 1996;126-135.
2. Wegener HC. Current aspects of foodborne salmonellosis. Proc Vol 1, 4th World Congress Foodborne Infections and Intoxications. 1998;102-106.
3. Wegener HC, Bagner F. Pork as a source of human Salmonella infections. Proc 2nd International Symposium on Epidemiology and Control of Salmonella in Pork. 1997;12-16.
7. Kasbohrer A, Blaha T. Field experiences with monitoring and reducing Salmonella in a poultry meat production chain. Proc 2nd International Symposium "Salmonella and Salmonellosis". Ploufragan, France. 1997.
8. Davies PR, Morrow WEM, Jones FT, Deen J. Salmonella in market-age swine in North Carolina. Proc 14th International Pig Veterinary Society Cong. 1996; 174.
9. Fedorka-Cray PJ, Bush E. Results of 1995 Swine survey in grower/finisher swine. Proc of the 100th USAHA Meet. 1996. Abstract.
10. Letellier A, Messier S, Quessy S. Prevalence of Salmonella spp. in finishing swine in Canada. Proc of the 2nd International Symposium on Epidemiology and Control of Salmonella in Pork. 1997;235.
11. Ganter M, Muller K, Tegeler R, Friedel K. Prevalence of Salmonella in finishing pigs of northwest Germany. Proc of the 15th IPVS Cong. 1998;70
