Diagnostic notes
Non refereed - This article has not been peer reviewed.
Centers for Disease Control and Prevention, Fort Collins,
CO 80522. Tel: 970-215-3778;
E-mail: mbunning@afmic.detrick.army.mil.
Bunning M. Nipah virus outbreak in Malaysia, 1998-1999. J Swine Health Prod. 2001;9(6):295-299. Also available as a PDF.
Nipah virus outbreak in Malaysia, 1998-1999
Mike Bunning, DVM, MPH
Introduction
For the past several years we have been faced with a new group of diseases referred to by the Centers for Disease Control and Prevention (CDC) as 'emerging' infectious diseases. The definition by CDC not only encompasses diseases associated with previously unknown (novel) agents, but also known diseases that are 're-emerging' either spatially or temporally. In 1999, a novel virus in Malaysia was responsible for the deaths of over 100 people and the mandated destruction of more than 1.1 million pigs.1 This virus was given the name 'Nipah', after the village where the first viralisolate was obtained. The eradication of 1.1 million swine represented approximately 40% of the swine population within Malaysia in 1999. As a result of the depopulation efforts, over 800 of Malaysia's 1700 swine operations were put out of business.2
Recently, within the United States, we have seen the introduction of another 'emerging' infectious disease called West Nile, and we have watched helplessly as it has spread over the eastern seaboard unchecked, infecting people, horses, and a variety of birds. This latest outbreak should awaken us to the fact that these infectious agents are capable of moving from continent to continent with relative ease. These two emerging infectious diseases as well as othersunderscore the need for veterinarians to keep abreast of foreign animal diseases and the potential threat they pose to both domesticanimal production and our wildlife. This article will outline some of the more salient features of Nipah virus in swine, based on our investigation of the Malaysian outbreak.
Background
During October 1998, an outbreak of fatal encephalitis occurred among pig farmers living in a swine production area near the town of Ipoh, Malaysia (Figure 1). While the outbreak in Ipoh had ended by late February 1999, leaving at least five people dead, a similar encephalitic illness had begun among pig farmers and their families living to the south in the Bukeit Pelandok area of the state of Negeri Sembilan (Figure 2). At first, the illness was believed to be Japanese encephalitis. It was not until March 19, 1999, that it was discovered that several patients from both regions had been infected with a new paramyxovirus.
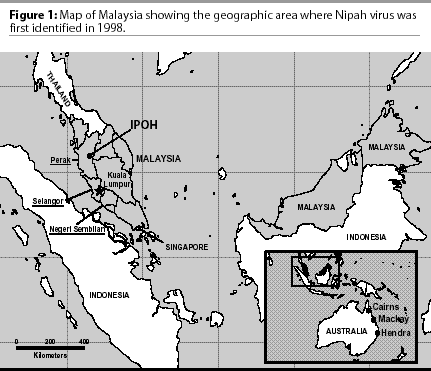
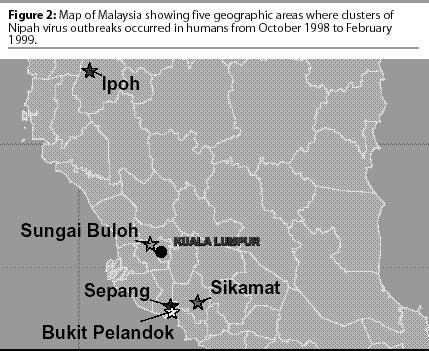
While the medical community attempted to unravel this new, mysterious disease, local and government veterinarians worked with the same pig producers attempting to identify a disease that was causing sickness in their pigs. The Malaysian government asked for international assistance, and by May, investigators from the United States, Australia, and Malaysia had determined that the same virus was indeed responsible for the illness identified in both the humans and the pigs.3
Etiology and host range
The infectious agent was identified as a new virus belonging to the Paramyxoviridae family. This same family of viruses is also responsible for measles, canine distemper, and Hendra virus -- another emerging infectious disease, first identified in Australia in 1994, which infectshumans, horses, and bats.4 Nipah virus has been shown through serology to infect people, pigs, horses, dogs, cats, chickens, and bats, but thus far, has only produced clinical disease in humans, pigs, dogs, and cats. There may be other animal hosts yet to be discovered.
Nipah virus is internationally classified as a biosecurity level 4 (BSL-4), the classification given to one of the world's deadliest viruses, Ebola. Simply put, these agents pose a risk to people during examination or treatment of infected animals; therefore, the examination and (or) necropsy of sick pigs requires specialized protective equipment (Figure 3). This protective equipment and the protocols involved when working with BSL-4 agents necessitates additional extensive training and constant vigilance by all personnel.

To date, Nipah virus has been identified only in Malaysia and has not re-emerged since the eradication program was completed in September of 1999. At the requestof the Malaysian government, CDC and Australia's Animal Health Laboratory (AAHL) and Animal Research Institute worked with Malaysian's own Department of Veterinary Services, Veterinary Research Institute, Institute Medical Research, Department of Medical Microbiology, University Malaya, and local health practitioners, in investigating and defining the outbreak.
Pathogenesis
Defining pathogenesis is one of the first priorities with any new disease, and in most cases, this aspect is first detailed in the field and substantiated later in the laboratory setting. Limited BSL-4 facilities exist throughout the world, and thus far AAHL has performed the only Nipah virus research in swine. The pathogenesis of Nipah virus infection in swine has not yet been completely defined.
The information presented is based on results of field investigations during the outbreak. Infection begins when the virus reaches the lungs of susceptible pigs, either through ingestion or inhalation. The incubation period is believed to be approximately 7 to10 days.5 Delineating the pathogenesis by examining field-collected specimens was potentially problematic due to the presence of concurrent porcine stress syndrome and infections such as Aujesky's disease (pseudorabies). Immunohistochemistry was used to confirm Nipah virus in histopathologic lesions. Lesions were identified in either the lungs or brains of infected pigs, or occasionally in both. Lung lesions consisted of tracheitis with bronchointerstitial pneumonia, both suppurative and nonsuppurative.3 It was assumed that the infection of the lung is responsiblefor spreading the virus to susceptible animals via direct contact or droplet infection. This was substantiated by work done by Middleton et al.5 All pigs with meningoencephalitis had some degree of meningitis, characterized by proteinaceous edema and infiltration of lymphocytes, plasma cells, and macrophages, and vasculitis, characterized by swollen vessel walls containing some macrophages.3
Clinical features
Nipah virus infects swine of all ages, but clinical appearances vary depending on age. Weaners and growers show an acute febrile illness with respiratory signs ranging from a mild cough to a harsh, "seal-like", nonproductive cough. Local swine producers referred to this cough as the "1-mile cough", as they claimed pigs could be heard coughing 1 mile away. Clinical signs varied with ambient temperature, with some animals exhibiting more severe degrees of respiratory distress, such as open-mouthed breathing, during the heat of the day. Some weaners and growers were obviously depressed, had a mucoid nasal discharge, and coughed persistently when forced to exert themselves. In my field investigation, I estimated that 3% of the animals showed some form of neurological disease, which included signs such as trembling, twitching, muscular spasms, and rear leg paresis or paralysis. The neurologic aspect of the disease appeared to be transitory, with most of this age group recovering within a couple of days.
Clinical presentation was quite different in adult swine. Most were found dead, with no previous indication of illness. In a few cases, we found sows in farrowing crates exhibiting tetanus-like spasms and seizures, chomping of the mouth, head pressing, and nystagmus. In these adult pigs, the disease progressed very rapidly and in most cases, death resulted. Abortions were reported by local producers, but were not observed by the field team.
The disease in suckling pigs is not well defined, probably because of decreased milk production by ill sows and (or) the diminished ability of ill piglets to nurse.
Case definition of Nipah infection developed by the investigation team
Serosurveys performed on a number of farms during this investigation indicated that morbidity approached 100%. Producers reported that the mortality rate due to Nipah virus infection was approximately 1 to 5%, but this was not substantiated.
Weaners (< 4 weeks old) and growers: Acute febrile illness (body temperature >=39.9 degrees C) with respiratory signs ranging from increased or forced respiration to harsh, nonproductive cough or open-mouth breathing. Respiratory signs may be accompanied by one or more of the following neurological signs: trembling, twitching or muscle fasciculation or tetanic spasms; rear leg weakness.
Adult pigs: Acute febrile illness (body temperature >=39.9 degrees C) with labored, open- mouth breathing, increased salivation, nasal discharge (possible bloody), and possible first trimester abortion. Sows and boars may die very rapidly (<=24 hours) with no signs of clinical disease. Some or all of the following neurologic signs may be present: head pressing; agitation or biting at bars; tetanic spasms.
Diagnosis
It is not possible to diagnose Nipah infection in swine solely on the basis of clinical examination, or gross or histopathologic appearance. However, strong suspicion of Nipah infection in swine can be made when area producers are ill with encephalitis, pigs have clinical disease consistent with the case definition, there is a history of movement of pigs between farms, and unexplained death of other farm animals occurs, specifically dogs and cats.
Disease transmission
The virus spread readily between grower pens separated by a 3- to 4-foot concrete walls and between unattached housing facilities, and continued to spread rapidly throughout the farm, infecting most of the pigs within a 2-week period. It is not known if the mechanism of disease transmission was direct contact or aerosol transmission from infected pigs, farm workers, or all of these. It is believed that the most likely means of virus transmission on a farm was direct contact or aerosol transmission between pigs.5
The spread of the disease within a farming community occurred by any number of the following methods, but has not been conclusively established: sharing boars or boar semen, moving pigs between farms or introducing newly purchased animals without a quarantine period, failing to properly disinfect feed trucks, movement of dogs and cats between farms, and movement of people between farms.
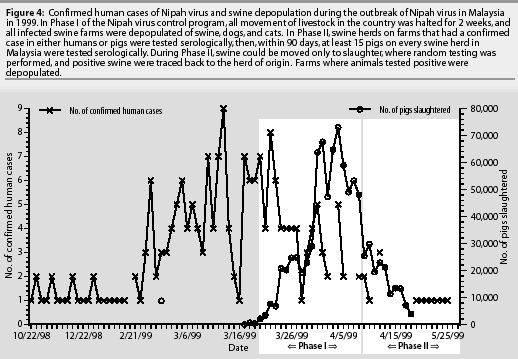
Liquidation of an infected pig farm in northern Malaysia was believed responsible for the movement of Nipah virus into other farming communities. As these infected pigs were moved from sparsely populated production areas in the north to dense farming areas in the south (Bukeit Pelandok, Sikamat farming communities), the spread of Nipah virus progressed rapidly from farm to farm (Figure 4).
Control
The Director General, Department of Veterinary Services, Malaysia, spearheaded a three-phase program, which was carried out and resulted in Office International Des Epizooties (OIE) declaring Malaysia Nipah-free early this year. The following is a brief description of this program.
Phase I. Movement of all livestock within the country was halted for 2 weeks. At the conclusion of the 2-week period, animals in non-infected areas were allowed to be transported only to slaughter facilities. Animals on farms having a confirmed human case or documented report of Nipah disease in pigs were immediately quarantined. Pigs on these farms, and all other animals on the premises, were depopulated, including dogs and cats. Farms within 3 kilometers of a positive farm were also quarantined. Police and military personnel secured the perimeter of these areas while all animals were depopulated. The farms were not permitted to restart swine production for an unspecified period of time, if ever. Random testing of swine in federally inspected slaughterhouses was implemented.
Phase II. The objective was twofold: within 90 days, to identify "high-risk" farms, and then to systematically test every pig farm in Malaysia for evidence of Nipah virus infection.
The "high-risk" farms were production facilities that had a new confirmed human case and (or) reported Nipah disease in swine. These farms were tested as a first priority to rapidly identify new foci of infection. Pigs from these high-risk farms were not allowed to be shipped for slaughter while further testing was carried out. Farms having laboratory evidence of Nipah disease were immediately depopulated.
The second objective was to test every pig farm in Malaysia for serologic evidence of Nipah virus. Each farm was tested twice within 90 days to ensure that every positive premise was identified as quickly as possible. A representative sample of pigs (at least 15 from each swine herd) were selected at random and tested for Nipah antibody. The two tests were separated by at least 2 weeks, and every effort was made not to test the same animals. Positive farms were immediately depopulated. All swine farms that were not identified as "high-risk" were provisionally approved to ship for slaughter only. Within Malaysia, swine could be moved only to slaughter. Transfer between farms was not permitted, nor was importation of new breeding stock.
During this phase, random testing continued at the slaughterhouse, and when positive animals were identified, they were traced back to the farm, where further testing was done.
Phase III. Upon completion of Phase II, active surveillance was continued by government veterinarians following up on reported, unexplained illness in swine or people. Random testing continued in federally inspected slaughterhouses. On the basis of the success the Malaysians experienced, there is no reason to believe that the United States would handle a similar outbreak any differently (Figure 4).
Current threat
The wildlife reservoir for Nipah virus is currently under investigation. On the basis of the work done in Australia on the closely related Hendra virus, bats, specifically pteropids, commonly called fruit bats (suborder Megachiroptera), are believed to be the reservoir.6 Keeping in mind the similarities between Hendra and Nipah viruses, the investigative team concentrated efforts on this reservoir. Specimens collected from five species of bats (four species of fruit bat and one insectivorous bat species) during the field investigation in Malaysia contained neutralizing antibodies to Nipah virus. Nipah virus has not been isolated from bat tissues collected in Malaysia.7
We do not know why Nipah appeared in Malaysia in 1998 or if it will return, but we have every reason to believe that another outbreak is possible. We cannot predict where it will appear, so we will need to add Nipah disease to the list of foreign animal diseases that we learned about early in our careers and hoped we would never see.
The outbreak in Malaysia has caused governments to reexamine their plans for animal depopulation and disease control. Prior to this outbreak, depopulating animals in a BSL-4 environment had not been considered. This outbreak has brought about many 'firsts' in disease control:
- Depopulation of animals was carried out in a BSL-4 environment.
- A National Control and Surveillance Program was rapidly developed and implemented.
- The military was responsible for depopulation in a BSL-4 climate.
- Depopulation of pets was included.
The impact of this outbreak on Malaysia's agricultural community, medical-veterinary professions, and economy cannot be conveyed within these few paragraphs. The efforts of so many people to deal with this devastating disease and the ultimate eradication are worthy of our highest praise.
Acknowledgements
Many people were involved with this investigation and I am deeply appreciative of their efforts and friendship and am very proud to have worked with them.
Malaysia: Norain Karim, Mohd Nordine Mohd Nor, Aziz Jamaluddin, Ong Bee Lee, Azri Bin Adzhar, Gan Chee Hiong, Sharihuddin Shamsudin, Johara Mohd-Yob, Mahendran Renganathan, Chandrasekaran Subramaniam, Jasbir Singh, Jamal Hassan, Raymond Choo Pow Yoon, Mah Choew Kong, Cheang Heng Toon, Sohayati Abdul-Rahman, Mahani Abdul-Hamid, S Chandrasegaram, Henry Too Hing Lee, SK Lam, Redsuan Ibrahim, Goon Swee Cheong, Lye Munn Sann, Cheong Yew Hoong, R Murugaya, Mohamad Taha Bin Arif, Tee Ah Sian, Devan Kurup, Marzukhi Md Isa, Chua Kaw-Bing, Chang Choong Chor, Narimah Awin, Mangalam Sinniah, Kew Siang Tong, Mohd Rani Bin Jusoh, Sng Kim Hock, CT Tan, K Thong Wong, Shalini Kumar, R Giritharan, Chan Chee Hoe, Rebecca Chin, Thean Bee Har, Ding Lay Ming, Marina Abdu Hamid, K Kanesan, K Rajendran, Lum Yin Woh, Sree Raman, Dr Thayaparan, Dr Sothy, Dr Chandran, Dr Letchuman, Dr Tong, Dr Teng, Dr Guna, Dr Zainab, Peter Loh, R. Goh, John Arokiasamy, Flora Ong, Mr. Samiun, Dr Wee, Dr Hanjeet Kaur
Australia: Peter Daniels, Hume Field, John White, Christopher Morrissy, Paul Selleck, Peter Hooper, Debrah Middleton, Bryan Eaton, Harvey Westbury,
CDC: Duane Gubler, Brian Mahy, Rima Khabbaz, Clarence James Peters, Thomas G Ksiazek, James Olson, Pierre Rollin, Sherif Zaki, James Mills, Stuart Nichol, Larry Anderson, William Bellini, Paul Rota, Bruce Kropp, Grant Campbell, Nick Karabatsos, Umesh Parashar, Patrick Stockton, Deborah Cannon, Kathy Veilleaux, Kent Wagoner, Joni Young, April Allman, Reginald Shaw, David Duty, Bruce Cropp, Rebecca Deavors, Ellen Peterson
WHO: Kevin Palmer, John Kobayashi
References -- refereed
1. Chua KB, Bellini WJ, Rota PA, Horcourt BH, Tamin A, Lam SK, Ksiazek T, Rollin P, Zaki S, Shieh W, Goldsmith C, Gubler D, Roehrig J, Eaton B, Gould A, Olson J, Field H, Daniels P, Ling A, Peters C, Anderson L, Mahy B. Nipah virus: a recently emergent deadly paramyxovirus, Science. 2000;288:1432-1435.
2. Office International Des Epizooties (OIE). Disease Information Bulletin; May 28, 1999;12(20).
3. Hopper P, Zaki S, Daniels P, Middleton D. Comparative pathology of the diseases caused by Hendra and Nipah viruses. Microbes Infect. 2001;3:315-322.
4. Selvey L, Sheridan J. Outbreak of severe respiratory disease in humans and horses due to a previously unrecognized paramyxovirus. Comm Dis Intell. 1994;18:499.
6. Murray PK, Eaton B, Hooper P, Wang L, Williamson M, Young P. Flying foxes, horses and humans: a zoonosis caused by a new member of the Paramyxovirdae. In: Scheld WM, Armstrong D, Hughes JM, eds. Emerging infections 1. Washington DC: ASM Press; 1998:43-58.
7. Yob JM, Field H, Rashdi AM, Morrissy C, van der Heide B, bin Adzar A, White J. Nipah virus infection in bats (Order Chiroptera) in peninsular Malaysia. Emerg Infect Dis. 2001;7:439-41.
References - non refereed
5. Middleton D, Westbury H, Morrissy C, vander Heide B, Russel G, Braum M, Muschialli J, Carlson D, Daniels P. Experimental Nipah virus disease in pigs: clinical features, virus excretion and subclinical infection. Proc 16th IPVS Cong. Ocean Grove, California. 2000;552.
