Listed in reverse chronological order by date of original publication; corrections have been made online when practical:
In the article on page 20 of the January/February 2023 issue of the Journal of Swine Health and Production (Little et al), the DOI for the Supplementary materials was incorrectly reported as “https://doi.org/10.54846/jshap/1297suppl.” The correct DOI for the Supplementary materials is https://doi.org/10.54846/jshap/1297suppl1
In the article on page 12 of the January and February 2020 issue of the Journal of Swine Health and Production (Menegat et al), the citation was incorrectly reported as “J Swine Health Prod. 2019;28(1);12-20.” The correct citation is J Swine Health Prod. 2020;28(1):12-20.
In the article on page 21 of the January and February 2020 issue of the Journal of Swine Health and Production (Free et al), the citation was incorrectly reported as “J Swine Health Prod. 2019;28(1);21-30.” The correct citation is J Swine Health Prod. 2020;28(1):21-30.
Radke SL, Olsen CW, Ensley SM. Elemental impurities in injectable iron products for swine. J Swine Health Prod. 2018;26(3):142-145.
In Table 1, the detected concentrations listed for chromium are correct, however, there is 1 cell shaded blue (exceeds the permitted daily exposure [PDE] by >25%) that should be shaded yellow (≤ 25% higher than the PDE) and 5 cells shaded yellow (≤ 25% higher than the PDE) that should be unshaded because they are ≤ to the chromium PDE.
In the article on page 29 of the January/February issue of the Journal of Swine Health and Production (Scherba et al), the citation was incorrectly reported as “J Swine Health Prod. 2016;24(1):21-28.” The correct citation is “J Swine Health Prod. 2016;24(1):29-35.”
In the article on page 198 of the July/August issue of the Journal of Swine Health and Production (Greiner), the citation was incorrectly reported as “J Swine Health Prod. 2015;24(4):198-204.” The correct citation is “J Swine Health Prod. 2016;24(4):198-204.”
Perri AM, Friendship RM, Harding JSC, et al. An investigation of iron deficiency and anemia in piglets and the effect of iron status at weaning on post-weaning performance. J Swine Health Prod. 2016;24(1):10–20
In the article on page 10 of the January and February 2016 issue of the Journal of Swine Health and Production (Perri et al), the citation was incorrectly reported as “J Swine Health Prod. 2015;24;10-20.” The correct citation is J Swine Health Prod. 2016;24(1):10-20.
Gamboa-Marín A, Mejía-Wagner DC; Paola A. Moreno-Ocampo PA, et al. Antimicrobial susceptibility of Listeria moncytogenes, Listeria ivanovii, and Listeria species isolated from swine processing facilities in Colombia. J Swine Health Prod. 2013;21:10–21.
In Table 2, the unit for the break points was incorrectly provided as “mg/mL.” The correct unit, “µg/mL,” now appears in the online edition.
Hughes PE, Smits J, Xie Y, Kirkwood RN. Relationships among gilt and sow live weight, P2 backfat depth, and culling rates. J Swine Health Prod. 2010;18:301–305.
In Figure 1, the bars were incorrectly labeled. For sow weight, black bars are Control sows and yellow bars are Culled sows. For sow P2 backfat, blue bars are Control sows and red bars are Culled sows. The figure on the right is correctly labeled.
Figure 1: Changes in body weight and P2 backfat depth (± SD) in relation to parity of gilts and sows culled or not culled (controls) from a 5400-sow commercial farrow-to-finish facility in New South Wales, Australia, during a 12-month period. Numbers of non-culled (control) females were 492, 847, 644, 1132, and 263 in parities 0, 1, 2, 3-5, and ≥ 6, respectively. Numbers of culled sows were 528, 243, 161, 586, and 636, in parities 0, 1, 2, 3-5, and ≥ 6, respectively. Body weight increased with increasing parity (P < .05; ANOVA) but backfat did not (P > .05) 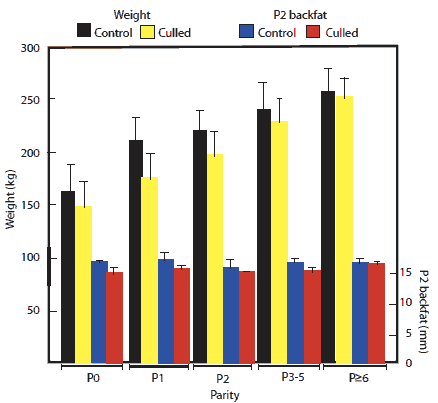 |
Jackson CJ, Karriker LA, Stalder KJ, Johnson AK. Number of visits and length of each visit to a nipple cup drinker by 7-week-old pigs after a water deprivation period or ad libitum access to water. J Swine Health Prod. 2009;17:76-80.
At the top of page 78, first sentence of the first paragraph, water flow rate was incorrectly reported as 29.9 L per second. The correct flow rate is 137 seconds per L.
In Table 3, cumulative SE for water disappearance was reported as 1.00. The correct value is reported in the following table.
Table 3: Least squares means and SE of water disappearance during a 5-hour observational period of 7-week-old pigs either after a 15-hour water withholding period (WH) or after ad libitum access to drinking water (C)*
* Trial described in Table 1. † ANOVA; pen nested within treatment and day was included as a random effect in the model, with body weight (kg) used as a linear covariate. |
|||||||||||||||||||||||||||||||||||||
Main RG, Dritz SS, Tokach MD, et al. Effects of weaning age on growing-pig costs and revenue in a multi-site production system. J Swine Health Prod. 2005;13:189-197
There is an error in the Figure 1 legend. The last sentence of the legend should read "In this modeled example, a 0.10-pig increase in total born on sow litters and a 0.05% increase in preweaning mortality are recognized for each 1-day increase in lactation length."
Mengeling WL. The porcine reproductive and respiratory syndrome quandary. Part II: Vaccines and vaccination strategy. J Swine Health Prod 2005:13:152-156.
The citation for Reference #9 was erroneously reported. The correct citation is as follows:
Opriessnig T, Pallares FJ, Nilubol D, Vincpent AL, Thacker EL, Vaughn EM, Roof M, Halbur PG. Genomic homology of ORF 5 gene sequence between modified live vaccines and porcine reproductive and respiratory syndrome virus challenge isolates is not predictive of vaccine efficacy. J Swine Health Prod. In press.
Yoo BW, Choi SI, Kim SH, et al. Immunostimulatory effects of an anionic alkali mineral complex solution (Barodon®) on porcine lymphocytes. J Swine Health Prod. 2002;10(6):265-270.
In Figure 1c, P values were incorrectly reported for weeks 11 and 13. The
correct P values are .02 for week 11, and .05 for week 13.
Figure 1d was incorrectly reported, and the correct graph is provided. The
proportions of non T/non B (N) cells were higher in the Barodon-treated group
than in the control group at Study Weeks 3 (P=.02), 11 (P=.02), and 13 (P=.03),
but not at Study Week 8.
Otake S, Dee SA, Rossow KD, Deen J, Joo HS, Molitor TW, Pijoan C. Transmission of porcine reproductive and respiratory syndrome virus by fomites (boots and coveralls). J Swine Health Prod. 2002;10(2):59-65.
The following errata were present in the original:
· The credentials of one of the authors, Dr John Deen, were erroneously
reported as "DVM, PhD, Diplomate ACVM". Dr Deen's correct credentials
are "DVM, PhD, Diplomate ABVP".
· In Figure 1, page 61, the letter designates on the diagrams for
the Alternative protocol and the Standard protocol were inadvertently reversed
on the right side of the figure. The designate for the Standard protocol (Group
4) should be the letter "C", and for the Alternative protocol (Group
5), the designate should be the letter "D".
Desrosiers R, Boutin M. An attempt to eradicate porcine reproductive and respiratory syndrome virus (PRRSV) after an outbreak in a breeding herd: eradication strategy and persistence of antibody titers in sows. 2002;10(1):23-25.
On page 24, last paragraph of the Results section, it was reported in error that spleen samples were tested by PCR: spleen samples were not tested.
On page 24, second column, third paragraph, the following sentence is inaccurate: "In a study by Wills et al, experimentally infectedpigs were able to transmit PRRSV for no more than 69 days." In the reported study, Wills et al found that "infected pigs were found to be contagious through day 62, but not after day 69."
Mathew AG, Beckman MA, Saxton AM. A comparison of antibiotic resistance in bacteria isolated from swine herds in which antibiotics were used or excluded. J Swine Health Prod. 2001;9(3):125-129.
References to Escherichia coli O157:H7 in this article were reported in error. In all instances, isolates were confirmed to be E coli O157, but testing for the H7 antigen was not performed.
Also in this article, the symbols ">=" and "<=" were inadvertently deleted during the printing process. The corrected information follows.
Materials and methods, page 126, second column: Resistance was determined at the following breakpoints: ampicillin, >=32 mg per mL; ceftiofur, >=8 mg per mL; gentamicin and oxytetracycline, >=16 mg per mL; and sulfamethazine, >=512 mg per mL.
Results, page 127, third paragraph: Resistance to ceftiofur tended to be low for all isolates, and even though differences occurred in MICs between the two herd types in isolates from the 4.5-kg pigs, all isolates were still within the range (<=2 mg per mL) considered to be susceptible to that drug (Figure 5).
Results, page 128, first paragraph: Approximately 2% of salmonellae from AU farms were determined to be resistant to oxytetracycline, on the basis of human-derived breakpoints (MIC>=16 mg per mL), whereas no resistant isolates were cultured from the AF herd.
Discussion, page 128, last lines: The greater MICs noted for ampicillin in E coli from younger animals were primarily due to larger numbers of resistant organisms with breakpoints >=128 mg per mL.
Hurd HS, Bush EJ, Losinger W, et al. Outbreaks of porcine reproductive failure: Report on a collaborative field investigation. J Swine Health Prod. 2001;9(3):103-108.
In this article, the symbol ">=" was inadvertently deleted during the printing process. The corrected information follows.
Results, page 106, second column, third paragraph: A sample was considered positive if the sample-to-positive ratio was >=0.4.
Table 2, footnote "a": HerdCheck(R) (ELISA), sample:positive ratio >0.4 considered positive.
Charles SD, Abraham AS, Trigo ET, et al. Reduced shedding and clinical signs of Salmonella Typhimurium in nursery pigs vaccinated with a Salmonella Choleraesuis vaccine. Swine Health Prod. 2000;8(3):107112.
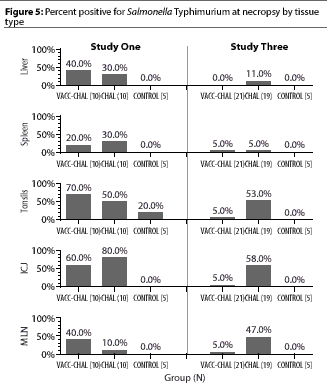
In Figure 5, page 111, the correct value for ICJ, Study 3, VACC-CHAL(21) is 5%, not 50%.
Amass SF, Clark LK. Biosecurity considerations for pork production units. Swine Health Prod. 1999;7(5):217-228.
Cleaning and disinfection, Vesicular exanthema virus, page 225: The disinfectants that inactivate this virus were reported in error. The correct information follows:
Blackwell93 reported VEV was inactivated after 2 minutes of exposure to 0.1% sodium hypochlorite, 2% sodium metasilicate, 5% sodium carbonate, 2% sodium hydroxide, 2% citric acid, 5% acetic acid, and 5% phenol.
Marsteller TA, Fenwick B. Actinobacillus pleuropneumoniae disease and serology. 1999; 7(4): 161-165.
Several errors were published in Table 1 (page 163). Biochemical and bacterial growth characteristics are important in the classification of bacteria. However, the enzymes used in biochemical reactions can be up-regulated or down-regulated by the bacteria and may cause confusion with bacterial classification. Polymerase chain reaction, which uses the DNA of bacteria, may be important in classifying any bacteria when confusion exists concerning bacterial biochemical reactions. Actinobacillus pleuropneumoniae is especially noted for its variable biochemical reactions.21 The corrected Table 1 appears here.

del Castillo, Jerome. Pharmacokinetic modeling of in-feed tetracyclines in pigs using a meta-analytic compartmental approach. 1998;6(5):189202.
Some text was inadvertently deleted from the bottom of page 195. The affected paragraphs should read:
As expected, these models were not able to predict plasma concentrations of OTC in fasted piglets dosed with a medicated drench.22,23 Plasma OTC concentrations found with medicated drench administration to fasted22,23 or fed22 pigs were considerably higher than both concentrations predicted with the equations and those from their experiments using medicated feeds.25,26 Intestinal absorption of OTC is reduced by the presence of food.5
Our model for CTC was also in close agreement to observations reported by Andrews, et al.18 in pigs that were challenged with Pasteurella multocida and then received CTC-medicated feed. This agreement is somewhat surprising, because the disease challenge is known to modify drug disposition in animals.1
Wu CC, et al., Testing antimicrobial susceptibility against Mycoplasma hyopneumoniae in vitro. 1997;5(6):227230.
In the results section (page 229), the MICs for Lincomycin were reported as <= 0.5 µg per mL for 93% of the isolates. This value is incorrect. The correct MIC for those 93% of the isolates is 1 µg per mL.
The value reported in the summary (page 227) is correct as it stands.
The author adds the following comment:
"We are aware of the extralabel prohibition against fluoroquinolones maintained by the CVM. Our intent in including this compound in our study was not to recommend to practitioners that a quinolone be used in swine for Mycoplasma, but rather to provide in vitro data to suggest further developmental work only."
Lane D, et al. Using partial budgets to analyze selected management practices associated with reduced preweaning mortality. 1997;5(3):95-102.
The capital recovery charges formula given on p. 97 is incorrect. The correct formula appears below:
![]()
where:
i = annual interest rate
n = number of years of useful life
BV = beginning market value
EV = ending market value
Bruna G, et al., Comparison of techniques for controlling the spread of PRRSV in a large swine herd. 1997; 5(2):5968.
On page 61, the vertical axis labels for Figure 2 (PRRSV serology) are incorrect. The prevalence of pigs at 21 days of age is 76.6% for those with antibody titers <16 and 6.6% for those with antibody titers of 16.The corrected figure appears here.
Davies PD. Food safety and its impact on domestic and export markets. 1997; 5(1):1320.
The values given in Table 4 (Codex, Japanese, and United States Maximum Residue Limits for oxytetracycline and chlortetracycline) were misreported. The values in the table should be [micro]g per kg, not mg per kg as stated.
Since the release of the January issue of Swine Health and Production, the United States Food and Drug Administration has published amended residue limits. The new limits are reported in Table 4.
Kirkwood, R, et al. The effect of dose and route of administration of prostaglandin F2[alpha] on the parturient response of sows. 1996; 4(3):123-127.
The results reported for the "late" sows (cumulative farrowing 8-48 hours after treatment) in Figure 2 were reported incorrectly. The correct values are as follows:
88 [micro]g V = 29.1%
88 [micro]g P = 33.8%
44 [micro]g V = 21.9%
44 [micro]g P = 22.3%
