Original research
Peer reviewed
Studies on survival of pseudorabies virus, Actinobacillus pleuropneumoniae, and Salmonella serovar Choleraesuis in composted swine carcasses
Josep Garcia-Siera, DVM, MS; Dale W. Rozeboom, MS, PhD; Barbara
E. Straw, DVM, PhD; Brad J. Thacker, DVM, PhD;
Larry M. Granger, DVM; Paula J. Fedorka-Cray, MS, MAS, PhD; Jeffrey
T. Gray, MS, PhD.
JGS: Indukern Corp, Miami, Florida 33178; DWR: Department of Animal Science, 2209 Anthony Hall, Michigan State University, East Lansing, Michigan 48824-1225; BES: Department of Large Animal Clinical Sciences, Michigan State University; East Lansing, Michigan 48824-1225; BJT: Department of Veterinary Diagnostic and Production Animal Medicine, Iowa State University, Ames, Iowa 50011; LMG: Animal Health Division, Michigan Department of Agriculture, Lansing, Michigan; PJFC, JTG: USDA-ARS-SAA, Richard Russell Research Center, Athens, Georgia.
Garcia-Siera J, Rozeboom DW, Straw BE, et al. Studies on survival of pseudorabies virus, Actinobacillus pleuropneumoniae, and Salmonella serovar Choleraesuis in composted swine carcasses. J Swine Health Prod. 2001;9(5):225-231. Also available as a PDF
Summary
Objective: To monitor survival of pseudorabies virus (PRV), Actinobacillus pleuropneumoniae (App), and Salmonella serovar Choleraesuis (Sc) in composted swine carcasses.
Methods: In Experiment One, pigs were infected with PRV, infected 2 days later with App, and euthanized 15 to 16 hours after App infection. Carcasses were then composted for 35 days. In Experiment Two, pigs were infected with Sc and euthanized 3 days later, and carcasses were composted for 10 days. Compost piles were constructed inside buildings with concrete floors. In both experiments, temperature of the composting piles was monitored daily, and samples were obtained from the carcasses for microbiologic evaluation at intervals throughout the composting period.
Results: Temperature of the composting piles ranged from 27 to 51 degrees C in Experiment One, and 27 to 62 degrees C in Experiment Two. Composted carcasses degraded rapidly. After 7 days, only bones, teeth, large muscles, and portions of the hide were physically recognizable. Muscle and bone were discolored, and bones were more easily crushed or broken. The hide was less collagenous and tore into several pieces when carcasses were extracted from piles. In Experiment One, tissue samples collected on Composting Days 7 and 14 were culture negative for PRV and App. In Experiment Two, Sc was recovered from samples collected on Composting Days 0, 1, and 3, but not from samples collected on Days 7 or 10.
Implications: Under the conditions of these experiments, composting can be used to dispose of swine carcasses containing PRV, App, and Sc.
Keywords:  swine, composting,
pseudorabies, Actinobacillus pleuropneumoniae, Salmonella
serovar Choleraesuis
swine, composting,
pseudorabies, Actinobacillus pleuropneumoniae, Salmonella
serovar Choleraesuis
Received: December 8, 2000
Accepted: March 11, 2001
In recent years, swine producers have been challenged with the increasingly difficult task of disposing of dead pigs and placentas on the farm. Incineration, burial, and rendering are frequently the only options. Incineration destroys pathogens, but has economical, environmental, and aesthetic drawbacks. Burial is cheap, but not always convenient in cold climates where the ground is frozen during winter. Rendering recovers several animal by-products and effectively controls transmissible diseases; however, not all producers have access to this method of disposal because of logistics or the size of the farm. Frequently, swine operations of 100 to 200 sows do not produce enough dead animals to justify service by a rendering plant. Also, unweaned pigs and placentas have no value for rendering companies and are not collected for disposal. In addition, rendering vehicles may serve as potential vectors of disease as they travel from herd to herd.
Composting initially gained popularity during the 1970's, largely because the Environmental Protection Agency sponsored development of specific composting techniques by the US Department of Agriculture, primarily to treat municipal waste water sludge or for solid waste management (sewage sludge). Presently, composting is most widely used to dispose of yard waste. Recently, however, interest has been directed towards composting as a method of dead animal disposal.
In the US, composting of dead animals has been confined primarily to poultry farms, with fewer swine farms making use of this practice. In several states, composting is not an approved method of carcass disposal. Experimental evidence supporting its usefulness and safety as a means of pig carcass disposal is lacking; therefore, regulations and laws governing pig carcass and placenta composting have not been established.
Few reports exist on survival profiles of microorganisms in composting piles. Flegal et al1 reported destruction of hemolytic enteritis virus in poultry composting piles. Morrow et al2 reported partial destruction of salmonellae and total destruction of Erysipelothrix rhusiopathiae and pseudorabies virus (PRV) by the heat produced in swine composting piles (internal temperatures in excess of 60 degrees C). In that study, salmonellae and Erysipelas organisms were grown in culture tubes that were sealed and buried in compost piles. Nine of 15 salmonellae culture tubes were culture negative when retrieved on day 127 of composting, and on day 177, 11 of 14 tubes were culture negative. All 18 Erysipelas tubes were culture negative when withdrawn from the compost pile on days 245 and 351 (9 tubes on each day). In the same study, tonsils from PRV-infected pigs, in sterile wide mouth glass bottles, and carcasses of four PRV-infected pigs, securely wrapped in plastic biohazard bags, were placed in compost piles. After extraction from the compost pile on days 29 and 53, tonsillar tissue samples from bottles and bagged carcasses were placed in a common scintillation vial, stored frozen for an unspecified length of time, and used to prepare an inoculum which was injected into PRV-free sentinel pigs. At the end of a 28-day observation period, serum samples collected from the pigs were negative for PRV antibodies.
While results from Morrow et al2 suggest that pathogenic organisms may be inactivated by composting, the conditions of the trial did not reflect an "on-farm" situation. In their experiment, the pathogenic microorganisms were not directly in contact with the compost material, and conditions inside the culture tubes may not have adequately reflected those surrounding an infected carcass in a compost pile. The study reported here investigated the effect of composting the carcasses of experimentally infected pigs under simulated swine farm conditions in late spring to summer, in a structure with a roof and impermeable floor.
Materials and Methods
Experiment One, Part A: Composting and microbiological testing of carcasses infected with PRV and App
Experimental design
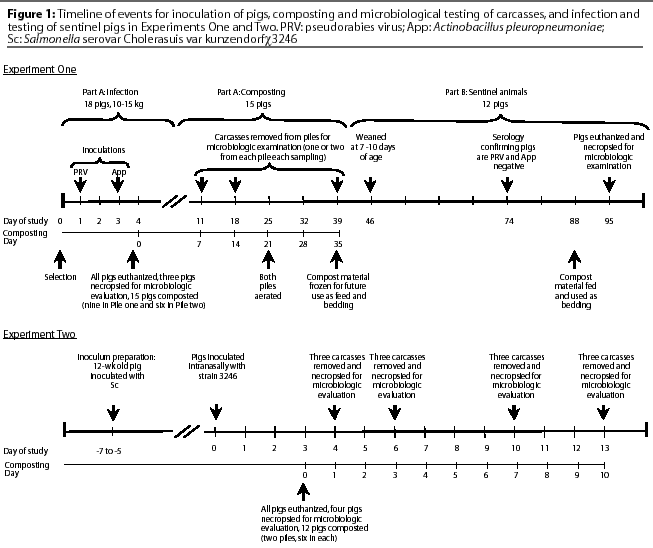
The sequence of events followed in Experiment One is shown in Figure 1. Eighteen pigs, weighing approximately 10 to 15 kg, were infected intranasally with PRV. Two days later, the pigs were inoculated intratracheally with App. All pigs were euthanized by intravenous injection of sodium pentobarbital 15 to 16 hours post challenge with App, when severe signs of App and (or) PRV had developed (fever, lethargy, inappetence). Lung and brain tissue from three carcasses (controls) were collected immediately after euthanasia (Composting Day 0) to determine the presence of PRV and App. The remaining 15 whole carcasses were placed in compost piles and tested for viral and bacterial survival after composting for 7, 14, or 35 days.
Challenge inocula and procedures
The strain of PRV used in this study was prepared as previously described.3 A 0.5-mL dose of challenge inoculum, containing 106 Tissue Culture Infective Doses (TCID) of PRV per mL, was instilled into each nostril over a 30-second period.3 Infectivity of the inoculum was confirmed by titration using the Crandall-Reese Feline Kidney (CRFK) cell line.
An App serotype 1 strain (Shope) was prepared for intratracheal challenge as previously described4 and contained 10 colony forming units (CFU) of App in 10 mL of normal saline. Pigs were anesthetized by IV administration of a mixture of ketamine hydrochloride (Ketaset; Fort Dodge Laboratories, Ft Dodge, Iowa), 4.4 mg per kg BW, and xylazine hydrochloride (Rompun; Miles Inc, Shawnee, Kansas), 1.65 mg per kg BW. The inoculum was placed in the trachea via percutaneous injection using an 18-gauge spinal needle. The inoculating dose was confirmed by back titration on brain-heart infusion agar (BHI; Difco Laboratories, Detroit, Michigan ) containing V factor (nicotinamide adenine dinucleotide; Sigma, St Louis, Missouri) as previously described.4
Compost pile construction,
temperature, and aeration
Two compost piles were constructed in an isolated, environmentally controlled building at the Michigan State University Veterinary Research Farm. Air temperature inside the building was maintained at 23 to 27 degrees C. One pile was placed in each of two rooms (3.1 x 4.3 m) with concrete block walls and concrete floors.
Pile One consisted of three layers of carcasses (C), one layer of straw (St), and four layers of fresh sawdust (Sd) free of foreign material and received directly from a saw mill that cut dried hard- and softwood lumber. Straw was used as the bottom layer to provide porosity and maintain aerobic conditions longer. The layout of the pile from bottom to top was St-Sd-C-Sd-C-Sd-C-Sd, with each layer approximately 15 cm deep. Strata in Pile Two were the same as in Pile One, but there were only two layers of carcasses. In each pile, a layer of carcasses contained three whole pigs. Total carcass weight was 92 kg for Pile One and 63 kg for Pile Two. The internal temperature of the compost piles was recorded with a 90-cm, probe-type thermometer. Temperature measurements were taken every 1 to 3 days by randomly inserting the probe approximately 30 to 45 cm into several areas of the pile. Early in the composting process, carcasses were struck with the probe and prohibited complete insertion. The thermometer was then inserted adjacent to the carcasses. After approximately 7 to 10 days of decomposition, the probe passed through carcasses. Piles were intentionally aerated on Day 21, after pile temperatures had peaked. Contents were manually turned and mixed using a garden shovel and fork. As a consequence, each pile was moved from its original location to a spot immediately adjacent. Prior to this, piles were aerated to an unknown but lesser extent by the random temporary removal of carcasses from their respective piles when samples were obtained.
Microbiologic techniques
Animal tissue or compost material was collected on Composting Days 0, 7, 14, and 35 to test for presence of infective microorganisms. Carcasses were randomly designated for sampling on Days 7 and 14. On Day 7, three whole pigs or their remains were extracted from each pile. One 15-g tissue sample was collected from the brain of each pig for PRV isolation, and another 15-g sample was collected from the lungs for App. On Day 14, carcasses were severely decomposed and individual soft tissues were no longer recognizable. Samples were collected near the intended anatomic location, which was determined by finding pieces of skin, hair, and large bones. Samples were thus composed of a mixture of animal tissue and compost material. Because of further decomposition and the aerating process, Day 35 samples were also a mixture of unrecognizable animal tissue and sawdust and could not be attributed to specific carcasses in either pile. Pile One samples may have contained animal tissue from both previously sampled carcasses and the three carcasses not sampled previously. Day 35 samples from Pile Two contained tissues from the six previously sampled carcasses. After collection, all samples were immediately placed in an ice-filled container and transported to the laboratory for processing.
Pseudorabies virus in brain tissue and compost samples was detected by virus isolation at the Iowa State University Veterinary Diagnostic Laboratory, Ames, Iowa, using a procedure previously described by Cook et al5 with the following modifications. For sample preparation, approximately 5 g of brain or compost sample was mixed with 20 mL of Earles balanced salt solution containing a fungistat and antibiotic (amphotericin B and gentamicin sulfate, respectively; Grand Island Biologics Co, Grand Island, New York). The mixture was placed in a stomacher 80 bag (Tekmar, Cincinnati, Ohio), blended for 20 to 25 seconds, and then centrifuged for 20 minutes at 1300g (Model TJ6; Beckman, Palo Alto, California). The supernatant was collected and 0.2 mL was inoculated onto Maden-Darby Bovine Kidney (MDBK) cell culture monolayers in 24-well microtiter plates. Cell cultures were observed for cytopathic effect for 7 days. Negative cultures were subcultured on MDBK cells for 7 more days.
Lung tissue collected on Day 0 for App isolation was processed as previously described.4 For lung tissue collected from composted carcasses and compost material, App isolation was performed by standard methods at the Iowa State University Veterinary Diagnostic Laboratory, Ames, Iowa, with the following modifications. Approximately 3 g of each sample was soaked in 30 mL of phosphate buffered saline solution prior to inoculation in triplicate onto 5% blood agar plates with Staphylococcus epidermidis feeder colonies. One plate was incubated aerobically, one plate in an atmosphere containing 5% CO2, and one plate anaerobically. Plates were incubated for 18 to 24 hours at 37 degrees C. In addition, a second culture was attempted after overnight enrichment of 2.5 g of each sample in 25 mL of Pleuropneumonia-Like Organism broth (PPLO; Difco), at 37 degrees C in an atmosphere containing 5% CO2.
Experiment One, Part B: Sentinel animal testing
Sentinel animals
Twelve pigs were weaned at 7 to 10 days of age. Segregated early weaning (SEW) was used to avoid dam-to-offspring transmission of PRV, App, or other pathogens that might interfere with the study. The herd of origin was tested monthly for PRV, had no history of PRV or App, was seronegative for PRV by ELISA (S:P<0.7) and latex agglutination tests, and was seronegative for App by complement fixation test (titer<1:8). Blood samples obtained from the 12 sentinel pigs at 5 weeks of age were negative for antibodies to PRV and App. Latex agglutination and ELISA tests were used to test for antibodies to PRV (Michigan Department of Agriculture Diagnostic Laboratory, East Lansing, Michigan) and an indirect ELISA was used to detect antibodies to App (Oxford Labs, Worthington, Minnesota; S:P>0.4 considered positive).
Housing and feeding
Pigs were housed in an environmentally controlled isolation facility, in a single pen (2.54 x 3.55 m) with solid concrete flooring, two nipple waterers, and a three-space, fence line feeder. Throughout this experiment, pigs were provided with ad libitum access to non-medicated feeds and water. From weaning to 4 weeks of age, pigs were fed two commercially available, pelleted diets for early-weaned pigs (EW and HE; United Feeds, Sheridan, Indiana) according to manufacture's feeding directions. After 4 weeks of age, the pigs were fed ground corn-soybean meal-based diets manufactured at the Michigan State University Feed Mill. The phase three diet (1.25% lysine, containing dried whey and fish meal) was fed from 4 to 6 weeks of age, and the phase four diet (1.15% lysine) was fed from 6 to 8 weeks of age. All diets met or exceeded NRC (1998) nutrient recommendations for pigs of like maturities.
Experimental design
The sequence of events followed in this experiment is shown in Figure 1. Potential exposure of pigs to PRV and App was accomplished by mixing material obtained from compost piles (Experiment One, Part A) into the feed, and using the same compost material as bedding. Both practices began when pigs were 7 weeks of age and continued for 7 days. Compost material had been stored frozen (-20 degrees C) for 7 weeks before it was blended by hand into the phase four diet (compost-to-feed ratio, 1:17) or used as bedding. Throughout the exposure period, pigs were monitored daily for signs of PRV and App infection, such as increased respiration rate, labored respiration, lethargy, and inappetence.
At the end of the feeding period (8 weeks of age), pigs were euthanized by IV injection of sodium pentobarbital. Brain tissue was collected for assay of PRV, and lung tissue for assay of App. Assays for PRV included immunofluoresence testing and virus isolation (MSU Animal Health Diagnostic Lab, East Lansing, Michigan) as previously described.3 Isolation of App from lung tissue was performed at the Iowa State University Veterinary Diagnostic Laboratory as described.
Experiment Two: Composting of carcasses infected with Sc
Bacterial strain, challenge culture, and preparation of inoculum
Wild type Salmonella serovar Choleraesuis var kunzendorf c32466 was kindly provided by the laboratory of Roy Curtiss III, Washington University, St. Louis, Medical Officer of Health to the National Animal Disease Center at Ames, Iowa. A 12-week-old pig was inoculated intranasally, and the isolate was recovered from the ileocolic lymph node (ICLN), stored at -70 degrees C in glycerol, and used as the challenge strain (3246pp).7 Strain 3246pp was streaked onto a trypticase soy agar (TSA) plate (Difco) which was sealed with parafilm and shipped to Michigan State University for preparation of challenge cultures. Sterile cotton-tipped swabs were used to prepare lawns of strain 3246pp on 20 TSA plates. After incubation of plates overnight at 37 degrees C, growth was harvested with a cotton- tipped swab and resuspended in 20 mL of phosphate buffered saline (PBS; 0.02M, pH 7.2). Final concentration of the inoculum was determined by plate count.
Experimental design
Figure 1 shows the sequence of events in Experiment Two. Sixteen pigs were housed in isolation facilities and challenged at 8 weeks of age (Study Day 0). The strain 3246pp inoculum, containing 2x1010 CFU per mL in PBS, was administered intranasally, 0.5 mL in each nostril dropwise on inspiration. Clinical signs of lethargy, anorexia, and dyspnea were monitored by observation on Days 1 through 3 while feeding the pigs. On Day 3 post challenge, all pigs showed various degrees of lethargy, anorexia, and dyspnea and were euthanized with sodium pentobarbital (IV). Necropsies were immediately performed on four pigs and 13 samples were collected per pig. Entire lymph nodes (LN) collected for bacteriologic culture included mandibular (mandib-LN), bronchial (bronch-LN), ileocolic (ICLN), and colonic (CLN) lymph nodes. Also collected for bacteriologic culture were 6- to 8-g samples of tonsil, lung, spleen, liver, middle ileum (ileum-mid), ileocolic junction (ICJ), cecum, and colon. Lastly, 25 g of cecal contents (CC) was collected and cultured.
The remaining 12 pigs were placed in two compost piles as described below. Necropsies were performed on three different pigs on each of Composting Days 1, 3, 7, and 10. Tissues were recognizable on Days 1 and 3, allowing for the collection of the desired 13 samples. All but two specific samples were obtained on Day 7. However, by Day 10, organs had severely decomposed, and unrecognizable tissue, lying in a similar anatomic location to the desired tissue or material, was collected.
Compost pile construction
Two compost piles were constructed, consisting of one layer of straw (bottom), one layer of spelt hulls, one layer of carcasses (six pigs), and one layer of spelt hulls (top). Each layer was approximately 20 cm deep. After the piles had been constructed, water was added to increase the moisture content to about 60%, based on the proximate analysis of the saw dust samples (ranging from 8 to 12% moisture)8 and the reported moisture content (about 45%) of young pigs.9 Each of the two sampling areas in each pile contained three carcasses.
Compost piles were contained in separate bays in an open front, naturally ventilated, multiple-bay pole barn, which had a roof and a concrete floor. Bays were approximately 3 x 5 m and had three solid wooden walls. The fourth side of each bay was closed with square straw bales so that compost piles measured approximately 1.5 x 3 m. This experiment was conducted in the spring, with daily low air temperatures ranging from -2 to 14 degrees C (Capitol City Airport, Lansing, Michigan; average, 7 degrees C) and daily high air temperatures ranging from 12 to 27 degrees C (Capitol City Airport, Lansing, Michigan; average, 19 degrees C).
Bacteriologic techniques
Tissues were processed according to the method described by Gray et al.7 Briefly, tissues collected at necropsy were minced using a sterile scalpel, then homogenized in a stomacher 80 laboratory blender (Tekmar, Cincinnati, Ohio). All tissues were incubated aerobically for 18 to 24 hours at 37 degrees C in GN-Hajna broth with streptomycin sulfate (200 µg per mL; GN-S; Difco), then 1 loopful was streaked on brilliant green agar with sulfadiazine (1 g per L) and streptomycin sulfate (200 µg per mL; BGS-S; Difco). Additionally, at 18 to 24 hours, 100 µl of GN-S suspension was transferred to Rappaport-Vassiliadis (RV) medium,10 incubated aerobically at 37 degrees C for 18 hours, then streaked on BGS-S. All BGS-S plates were incubated aerobically for 24 hours at 37 degrees C. Colonies having the appearance of Salmonella serovars were picked and inoculated into triple sugar iron and lysine iron agar slants. Isolates having biochemical reactions typical of Salmonella serovars were confirmed as group C by agglutination with Salmonella serovar antiserum group C1O (Difco). Representative isolates were serotyped at the National Veterinary Services Laboratories (NVSL; Ames, Iowa).
Bacterial counts were conducted on the ICJ samples using the five-tube most probable number method (Wood and Rose, 1992)11 with GN-S, BGS-S, and RV media as described above and reported as the mean value. Bacterial counts were also conducted on the ICLN samples by direct plate count using limiting dilution.
Results
Experiment One: Part A
On Composting Day 0, PRV was isolated from three of three brain samples, and App (>1000 CFU) was recovered from three of three lung samples, collected from the control pigs immediately after euthanasia.
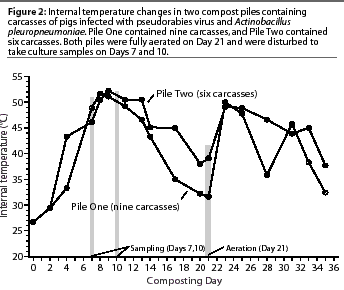 Temperature in compost piles ranged
from 26.7 degrees C (near ambient temperature in the building)
to 52.2 degrees C (Figure 2). The highest temperature in Pile
One (51.7 degrees C) was reached on Day 8 of composting, and the
highest temperature in Pile Two (52.2 degrees C) was reached on
Day 9 of composting. Temperatures in the piles remained above
45 degrees C until Day 14, then started to drop, more
rapidly in Pile One than Pile Two. On Day 23, 2 days after the
piles were aerated, the temperatures peaked again at 50 degrees
C.
Temperature in compost piles ranged
from 26.7 degrees C (near ambient temperature in the building)
to 52.2 degrees C (Figure 2). The highest temperature in Pile
One (51.7 degrees C) was reached on Day 8 of composting, and the
highest temperature in Pile Two (52.2 degrees C) was reached on
Day 9 of composting. Temperatures in the piles remained above
45 degrees C until Day 14, then started to drop, more
rapidly in Pile One than Pile Two. On Day 23, 2 days after the
piles were aerated, the temperatures peaked again at 50 degrees
C.
By Day 7, the first day of tissue collection, specific anatomic structures within carcasses were physically unrecognizable. A small amount of brain tissue in liquid form was obtained from the skull cavity of one pig in Pile One. All other samples collected on Day 7 were mixtures of animal tissue and sawdust. Carcasses appeared to be decomposed except for pieces of bone and hide. Collections on Days 14 and 35 consisted of a mixture of animal tissue, sawdust, and small pieces of straw. By Day 14, carcasses were totally unrecognizable, except for a few 15-cm pieces of hide. Piles were dark brown with small bone segments visually detectable. Some skulls were still recognizable on Day 14. By Day 35, few major bones were recognizable.
Microorganism survival
Viruses were not detected in compost samples taken on Days 7 and 14. Therefore, virus isolation was not performed on samples collected on Day 35. No App colonies were recovered from compost samples on Days 7, 14, or 35.
Experiment One: Part B
During the final week of the experiment, sentinel pigs consumed approximately 1.5 kg of feed and compost material per pig per day. The pigs did not develop signs related to infection with App or PRV, and all tissues collected at the end of the experiment tested negative for both microorganisms.
Experiment Two
Bacterial strains and challenge cultures
Salmonella serovar Choleraesuis 3246pp was isolated from 44 of the 52 samples collected on Composting Day 0. In all four pigs, Sc was isolated from the ICLN, ICJ, liver, mid-IL, colon, and CC; in three of the four pigs, Sc was isolated from the mandib-LN, bronch-LN, CLN, tonsil, lung, and cecum; and in two of the four pigs, Sc was isolated from the spleen. On Composting Day 1, Sc was not isolated from the ICJ or CC of one pig, but was isolated from the other 37 of the 39 sites sampled. On Day 3, Sc was isolated from all 39 sites sampled. However, on Days 7 and 10, Sc was not isolated from any sample.
The mean number of Sc recovered from tissue samples was 3.6 x 105 CFU per g of ICJ and 1.0 x 103 CFU per g of ICLN.
All isolates submitted to NVSL were confirmed by serotyping to be Salmonella serovar Choleraesuis var kunzendorf.
Discussion
Isolation of App and PRV from all three control pigs in Experiment One and Sc from all four control pigs in Experiment Two demonstrates that the infection procedures were successful. Therefore, the pathogen content in the carcasses of these experimentally infected pigs probably closely resembled that found in clinical cases of these diseases. Usually, young pigs, similar in age to those used in this study, and not mature swine, die from active infections with PRV, App, and Sc. Therefore, the pigs in these experiments are representative of animals that might be composted under clinical and commercial conditions.
Multiple small compost piles were used in these studies, both to facilitate sample retrieval without greatly disturbing the piles and to better represent conditions that would be experienced on the outside layers of larger piles. Microbial activity and destruction of potential pathogens harbored in carcasses are slower in the outer layers of piles, possibly because of the greater porosity.2 Use of small piles also made it easier to identify the location of composted carcasses. Piles were constructed inside a totally enclosed building in Experiment One and in an open-front structure in Experiment Two. Some states, including Michigan, Illinois, and Minnesota, presently require that on-farm composting be conducted in a facility with a minimum of concrete floor, bin walls, and roof.
Temperatures in our piles attained 40 to 50 degrees C for prolonged periods, indicating that conditions (porosity, moisture content, and carbon-to-nitrogen ratio) were conducive to microbial population growth. Different composting materials, also known as bulking agents or carbon sources, were intentionally used in Experiment One (sawdust) and Two (spelt hulls), with no apparent impact on the composting process. Both provided adequate amounts of carbon. The decision to use spelt hulls in Experiment Two was based on cost (none), availability, and producer interest in using this milling industry by-product.
Previously composted material may be used to inoculate compost piles to speed the composting process.12 In these experiments, temperatures in the piles rose quickly, suggesting that sufficient numbers of composting microbes must have been naturally present on the fresh bulking agents and the carcasses. When the trial was terminated on Day 35, both piles had internal temperatures higher than the ambient temperature, suggesting that the biological activity of the composting process was not complete.
These trials were completed in the spring when ambient temperatures
were between
-2 and 25 degrees C. The composting process would have been different
if the new piles had been constructed outdoors during winter,
as we have observed in on-farm composting demonstrations in Michigan
(unpublished data). During the winter, temperatures in new piles
are slower to rise, but gradually reach peak temperatures comparable
to those found in this study, provided that carcasses are not
frozen. Frozen carcasses compost poorly during cold weather, especially
if the pile is being constructed with new material and the carcasses
are 10 kg or greater in size. If carcasses are cold but not frozen
when added to an already active compost pile in winter, the onset
of microbial decomposition would be delayed only slightly, by
3 to 7 days depending on ambient temperature, until the heat of
the compost mass warms the carcasses. An active pile would already
be warm, with substantial microbial decomposition already occurring.
Small frozen pigs would probably thaw when added to an active
compost pile during winter months, and microbial decomposition
would probably begin within 7 to 14 days. These observations are
in agreement with those of other researchers.12
In this study, the conditions achieved in the composting process killed PRV and the two species of bacteria in the carcasses. Our results are in agreement with those of Morrow et al,2 who reported that composting temperatures effectively and quickly destroyed PRV. Similarly, Davies and Beran13 have shown that at 37 degrees C and pH 6 to 8, PRV outside the living host is inactivated at a rate of 0.6 log 10 per day. Failure to isolate PRV from tissue samples collected from composting carcasses indicates that PRV was destroyed by Day 7.
Failure to infect sentinel pigs with PRV by exposing them to composted material in feed or bedding appears to be a confirmation that the PRV was destroyed prior to Day 35 of composting. This conclusion is debatable, however, as the compost material was stored frozen at -20 degrees C for 7 weeks from the time it was taken from the pile until its use in feed and bedding. Previous research14 has shown that PRV in pig muscle and bone marrow is killed by freezing (-18 degrees C) for 35 days.
Actinobacillus pleuropneumoniae was killed within 7 days in piles that became active quickly, as indicated by the rapid rise in temperature. This outcome was expected, as Nicolet15 reported that App survives outside the body for only a few days, even when protected by mucus and organic matter. Results in the sentinel pigs could be seen as confirmation of pathogen destruction by Day 35 or earlier. However, drawing a conclusion about the survival in the compost material is questionable for App, as it was for PRV, because App was frozen and stored for 7 weeks before the sentinel pigs were exposed to it.
Isolation of Sc from Day 0 tissue samples confirmed that all pigs were infected prior to composting. The pattern of tissue colonization and the mean numbers of Sc recovered from tissue samples were similar to those described by Gray and others.7
Salmonella serovar Choleraesuis was destroyed in infected swine carcasses by composting for 3 to 7 days. This conclusion is consistent with the report of Forshell and Ekesbo,16 which states that Salmonella serovar Senftenberg and Salmonella serovar Typhimurium do not survive more than 7 days in composted swine manure. In contrast, the results of other studies suggest that some salmonella may survive the composting process much longer. Droffner and Brinton17 observed that Salmonella serovar Typhimurium Q survived 59 days during the composting of industrial sludge, even though temperatures of 60 degrees C were attained. Morrow and coworkers2 reported that salmonellae in culture tubes survived after more than 6 months in compost piles containing swine carcasses. In their experiment, salmonellae were grown in trypticase soy broth in culture tubes which were then sealed, creating nearly anaerobic conditions. When removed from the pile 127 days after the composting process began, 40% of the tubes contained live salmonellae, and 21% of the tubes still contained live salmonellae by Day 177. In Morrow's study, salmonellae were exposed to heat alone. These authors explained that by isolating the bacteria in culture tubes, they "underestimated the bactericidal effect of composting." Droffner and Brinton17 similarly concluded that the destruction of salmonellae "during aerobic composting is complex and not simply the result of thermal physical environment." In our experiment, Sc was exposed to other potentially inactivating factors, such as water, air, and other microbes and the by-products of their activity, explaining the negative culture results for Sc in all samples on Day 7.
The number of swine pathogens examined in this study was intentionally limited to three. We know of no other studies where the carcasses of infected swine have been composted to evaluate the survival of pathogens. There is much concern worldwide about the spread of prions associated with transmissible spongiform encephalopathies and of the foot-and-mouth disease (FMD) virus. There are no published reports of swine being infected by prions, possibly because the short life span of swine limits infectivity. The agents of transmissible spongiform encephalopathies are very difficult to destroy18 and would probably survive the composting process. Foot-and-mouth disease is caused by an aphthovirus which is easily destroyed at pH above 9 and below 6.19 The pH of compost piles containing swine carcasses may range from 5.5 to 7.2, depending on carbon source.2 Whether compost pH and other conditions within the pile would inactivate the FMD virus is not known.
Mature animals were not used in the present study primarily because of their inconvenient size. Because of the greater collagen content of the hide and the greater mineralization of large bones in older swine, microbial decomposition is slower to initiate and longer to complete.12 The rate of decomposition depends on pile management, environment, and whether or not the carcasses are cut open prior to composting. If carcasses are left intact or unopened, it may take longer for the composting process to destroy pathogens such as PRV, App, or Sc. However, once exposed to microbes, the rate of decomposition of muscle, internal organs, and other tissues would probably be similar, regardless of animal age.
Implications
- Conditions in composting piles are adequate to kill pseudorabies virus, Actinobacillus pleuropneumoniae, and Salmonella serovar Cholerasuis in the carcasses of pigs weighing 10 to 30 kg.
- Composting is a safe method of disposal of swine carcasses of this size.
Acknowledgements
The Michigan Pork Producers Association, The Sycamore Creek Watershed Project, and The Michigan Department of Agriculture provided funding for this research.
References -- refereed
2. Morrow WE, O'Quinn P, Barker J, Erickson G, Post K, McCaw M. Composting swine as a suitable technique for managing swine mortalities. Swine Health Prod. 1995;6:236-243.
3. Vilnis A, Sussman MD, Thacker BJ, Senn M, Maes RK. Vaccine genotype and route of administration affect pseudorabies field virus latency load after challenge. Vet Microbiol. 1998;62:81-96.
4. Jolie RAV, Mulks MH, Thacker BJ. Cross-protection experiments in pigs vaccinated with Actinobacillus pleuropneumoniae subtypes 1A and 1B. Vet Microbiol. 1995;45:383-391.
5. Cook DR, Hill HT, Kinker DR. Efficacy of a killed gpX deleted pseudorabies virus vaccine. Can J Vet Res. 1990;54:438-445.
6. Kelly, SM, Bosecker BA, Curtiss R, III. Characterization and protective properties of attenuated mutants of Salmonella choleraesuis. Infect Immun. 1992;60:4881-4890.
7. Gray JT, Fedorka-Cray PJ, Stabel TJ, Kramer TT. Natural transmission of Salmonella choleraesuis in swine. Appl Environ Microbiol. 1996;62:141-146.
8. AOAC Official Methods of Analysis. 16th ed. Gaithersburg, Maryland: Association of Official Analytical Chemists International; 1995.
9. Shields RG Jr, Mahan DC, Graham PL. Changes in swine body composition from birth to 145 kg. J Anim Sci. 1983;57:43-54.
10. Vassiliadis P. The Rappaport-Vassiliadis (Rv) enrichment medium for the isolation of salmonellas: an overview. J Appl Bacteriol. 1983;48:167-174.
11. Wood RL, Rose R. Populations of Salmonella typhimurium in internal organs of experimentally infected carrier swine. Am J Vet Res. 1992;53:653-658.
13. Davies EB, Beran GW. Influence of environmental factors upon the survival of PRV. Res Vet Sci. 1981;31:32-36.
14. Durham PJK, Gow A, Poole WSH. Survival of Aujeszky's disease virus in frozen pig meat. Res Vet Sci. 1980;28:256-258.
15. Nicolet J. Actinobacillus pleuropneumoniae. In: Leman AD, Straw BE, Mengeling WL, D'Allaire S, Taylor DJ, eds. Diseases of Swine. 7th ed. Ames, Iowa: Iowa State University Press; 1992:401-408.
16. Forshell LP, Ekesbo I. Survival of Salmonellas in composted and not composted solid animal manures. J Vet Med. 1993;40:654-658.
17. Droffner ML, Brinton WF. Survival of E. coli and Salmonella populations in aerobic thermophilic composts as measured with DNA gene probes. Zbl Hyg. 1995;197:387-397.
18. Brown P, Rau EH, Johnson BK, Bacote AE, Gibbs Jr, CJ, Gajdusek DC. New studies on the heat resistance of hamster-adapted scrapie agent: Threshold survival after ashing at 600 degrees C suggests an inorganic template of replication. Proc Natl Acad Sci USA. 2000;97:3418-3421.
References -- non refereed
1. Flegal CJ, Gerrish J, Schwartz LD, Maes R, Endres F. Animal Protein Composting - Final Report. Michigan State University, 1993. Poultry Research Report.
12. Fulhage C. Composting dead swine. University of Missouri; 1994. Extension Publication WQ225.
19. AVIS. Consortium consisting of the Institute for Animal Health, UK; The Food and Agriculture Organisation, Rome; L'Office International des Epizooties, Paris and Telos; ALEFF Ltd., UK. Available at: http://www.aleffgroupl.com/avisfmd/A010-fmd/mod0/0123-ph.html . Accessed June 28, 2001.
