Literature review
Peer reviewed
A review of some aspects of the epidemiology, diagnosis, and control of Mycoplasma hyopneumoniae infections
Robert Desrosiers, DVM, Dipl ABVP
Boehringer Ingelheim (Canada) Ltd, 5180 South Service Road, Burlington, Ontario, Canada, L7L 5H4
Desrosiers, R. A review of some aspects of the epidemiology, diagnosis, and control of Mycoplasma hyopneumoniae infections. J Swine Health Prod. 2001;9(5):233-237. Available as a PDF
Summary
Mycoplasma hyopneumoniae (MH), one of the most important swine pathogens, is distributed world wide. Alone, MH is relatively benign,but associated with other pathogens, which is almost always the case in field situations, it may cause serious losses. The combination of porcine reproductive and respiratory syndrome virus and MH is particularly important in respiratory problems of grow-finish pigs. Different strains of MH exist, but their significance remains to be evaluated. Transmission occurs mainly by direct contact and aerosol. Cranioventral lesions of pneumonia are suggestive of MH, but are not pathognomonic. The use of modern production technologies has coincided, to a certain extent, with an upsurge of MH-associated problems. Antimicrobials are more efficacious when used for prevention rather than treatment. Optimal timing for vaccination depends on the age at which pigs will come in contact with the organism and their level of maternal immunity when vaccinated. It is possible to produce MH-negative pigs from infected herds and to eradicate the organism without total depopulation.
Keywords:  swine, Mycoplasma
hyopneumoniae, epidemiology, diagnosis, control.
swine, Mycoplasma
hyopneumoniae, epidemiology, diagnosis, control.
Received: July 11, 2000
Accepted: April 17, 2001
Mycoplasma hyopneumoniae (MH) is present in all countries of the world where a significant swine industry exists. The very high prevalence, coupled with associated losses, makes this organismone of the most important for swine veterinarians and producers. Furthermore, although MH has been recognized as a swine pathogen for decades, it appears that new production methods and possibly association with other agents, such as porcine reproductive and respiratory syndrome virus (PRRSV), have given it a new significance. This paper will briefly review some elements concerning its epidemiology, diagnosis, and control.
Epidemiology
Strain variability of mycoplasmas
Limited research indicates that antigenic and genetic variation does exist among MH strains. Heterogeneity was detected by Ro et al1 using a metabolic inhibition test, by Frey etal2 using restriction enzyme digestion and field inversion gel electrophoresis, by Artiushin et al3 using polymerase chain reaction (PCR), and by Lin et al4 using total protein profile, glycoprotein profile, and size differences in the amplified PCR products of field strains of MH. Lloyd et al5 inoculated groups of pigs intravenously with five different strains of MH, and found differences in clinical disease (numbers of pigs and joints with arthritis) and immune response. Although this study did not model respiratory disease,the results suggest that virulence differences may exist among MH strains, as they do among strains of Mycoplasma hyorhinis, another mycoplasma of pigs.6
Several species of pathogenic mycoplasmas, for example, M hyorhinis, Mycoplasma bovis, and Mycoplasma gallisepticum, have sophisticated genetic machinery for altering their surface characteristics by mechanisms such as phase variation, size variation, and epitope masking and demasking of surface proteins.7,8 It remains to be determined whether these mechanisms are also characteristic of MH, and to what extent they may explain apparent genetic and antigenic differences between strains.
Asymptomatic carrier pigs
It is unknown how long pigs and sows remain carriers of MH. In a recent study by Calsamiglia et al,9 nasal swabs tested by PCR were positive in sows up to their seventh parity. There was no correlation betweensow and piglet colonization, possibly because there were few positive piglets. Thacker10 reported that it is possible that MH is never completely eliminated from the respiratory tract. Mattsson et al,11 usingPCR, detected MH on nasal swabs from five of 31 pigs after 12 weeks in a finishing unit, while only one was positive 3 weeks later. Sørensen12 et al were unable to identify MH on nasal swabs from 200 experimentallyinfected pigs 81 days post infection (PI) either by PCR or isolation, although the organism could be readily isolatedfrom lung tissue.
Several studies have shown that it was possibleand even relatively easy to eradicate MH from infected herds by using a three-step protocol that included a break in farrowings, treatment with antimicrobials, and removal from the farm of all animals 10 months of age or younger, on the assumptionthat MH is mainly maintained in swine herds by young animals.13-15
Aerosol transmission
Pigs can be infected either by their dam or by other infected pigs on the premises. Airborne transmission occurs over short distances inside the farm, and the organism has been identified by PCR in the air of infected barns.16 The question of between-farm airborne transmission is still debated. In the mid-80s, Goodwin17 found that the risk of a herd becoming infected with MH was inversely related to the proximity of other pigs, and that, for maximum survival of MH-negative status in a herd, the crucial distancefrom an infected pig farm was approximately 3.2 km, suggesting that the airborne route was the most probable manner of infection. Muirhead et al18 reported that if there is an uninterrupted view of an MH-infected herd from a farm, particularly if it is less than 3 km away, it is likely that this farm will eventually become contaminated by wind-borne infection.
Results of a study by Thomsen et al19 in 204 specific pathogen free (SPF) herds in Denmark supported the theory that airborne transmission and contact with purchased, subclinically infected animals are major sources of MH infections, and showed that the risk of infection was greater for SPF herds close to a large infectedherd than for those close to a small infected herd. Results of an epidemiological study in Switzerland also supported the theory of airborne transmission.20 Researchersfound that factors associated with infection of Swiss SPF herds included the size of the herd, its distance from the nearest road used regularly by pig transporters, and its distance from the nearest infected herd. A seasonal pattern was identified, with most infections occurring betweenNovember and March. The theory of airborne transmission of MH is supported by the survivability of MH for at least 31 days in water at temperatures of 2 to 7°C.17
Transmission through semen
A few studies have examined the possibility of MH transmission by semen. In an early study in Finland,21 one of 101 semen samples was positive for MH. In a subsequent study in Denmark, 22 MH was not recovered from any of 169 semen samples tested. Semen is unlikely to be a significant concern in field situations.
Diagnosis
Clinical and pathological aspects
Mycoplasma hyopneumoniae causes enzootic or mycoplasmal pneumonia. Experimental infection of pigs with MH alone usually produces coughing, with little or no impact on performance.23 The pigs do not die and usually recover spontaneously. The purple to gray lesions of consolidation are invariably located in the cranioventral part of the lungs, extending caudally as severity increases.24Although gross lesions are suggestive of enzootic pneumonia, they are not pathognomonic for MH, as other organisms may produce very similar lesions.10,24 Consolidation regresses by 60 to 100 days PI.12 Enzootic pneumonia is primarily a problem of grow-finish pigs, but pigs as young as a few weeks of age or adult pigs may be affected, particularly in a recently infected herd.18,24
Although it has rarely been considered a significant cause of pericarditis, MH was isolated from the pericardium in 33 of 46 cases of pericarditis detected at slaughter in a recent study in Denmark.25 The authors concluded that mycoplasmas, and particularly MH, are the most likely cause of fibrinous pericarditis in slaughter pigs.
The mild disease associated with experimental MH infections contrasts with the severe outbreaks sometimes observed in field situations. The difference occurs mainly because other pathogens present on the farms contribute to the problem, sometimes causing, in addition to the cough that is usually present, dyspnea, fever, reduced growth, and increased mortality.10,26 More severe disease occurs when pigs are experimentally infected with both MH and Pasteurella multocida, compared to MH alone,27,28 and this appears to be true as well when MH is coupled with Actinobacillus pleuropneumoniae or the pseudorabies virus.29,30 Thacker et al31 have shown that MH infection may potentiate the disease and lesions induced by PRRSV.
Recently, "porcine respiratory disease complex" (PRDC) and the "18-week wall" have become familiar expressions, describing relatively new problems observed in finishing units, in which MH and PRRSV appearto play a significant role.10,26,31,32 Even in production systems using strategies believed to improve the overall health status of pigs (eg, all inall out pig flow, multiple sites, early weaning),24,26 these problems may be severe, and perhaps particularly so, possibly because few pigs come in contact with MH prior to being placed in the finishing units. These pigs constitute a large population of nonimmune animals. In traditional systems, where pigs were weaned at an older age and raised in continuous flow in the farrowing and nursery units, they were more likely to be exposed to MH while still under the partial protection of maternal immunity, and might have been better able to resist infection.26 Furthermore, PRRSV has become a constant concern since the late 1980's to early 90's. It is clear that the frequent association of PRRSV and MH in field situations has created a condition that is much more difficult to deal with than either disease individually.10,26,31-33
Laboratory diagnosis
Enzootic pneumonia is characterized by the accumulation of lymphocytes in perivascular, peribronchial, and peribronchiolar tissues.24 In a study by Sørensen et al,12 200 naive pigs were infected by aerosol. The mean onset of coughing was 13 days PI, but several pigs coughed as early as 6 days PI, and maximum coughing occurred 27 days PI. Lesionswere maximal 28 days PI, and were virtually undetectable 85 days PI. On day 14 PI, MH was detected in almost all lungs examined by cultivation, PCR, enzyme linked immunosorbent assay (ELISA), and indirect fluorescent antibody (IFA). By day 85 PI, cultivation was the most sensitive technique for detecting MH in the lungs. Nasal swabs were positive by cultivation in 1% of pigs and by PCR in 18% of pigs 25 days PI. However, all nasal swabs were negative by both techniques on day 81. The first pig became seropositive on a monoclonal antibody blocking ELISA 8 days PI. On average, the pigs became seropositive22 days PI, and all pigs were seropositiveby day 46. Four pigs retained to evaluate the duration of antibody titers still had positive titers 8 months PI.
Using the same ELISA, Rautiainen et al34 recently reported that MH antibodies persisted 3 years or more in some sows. In this study in Finland, frozen colostral samples were also tested in 530 herds to document freedom from infection, and the authors concluded that it was both a sensitive and convenient method of monitoring herds for MH.
In a field study by Sørensen et al,35 using the same ELISA, two groups of seronegative pigs became serologically positive an average of 6 and 9 weeks after being introduced into two different positive herds.
Using the same ELISA, Andreasen et al36 were able to detect maternal antibodies in piglets up to 7 weeks of age. Vaccination of sows before farrowing may increase the time that maternal antibodies will be detected in piglets. Thacker et al37 vaccinated sows three times prior to farrowing, and piglets of these sows remained seropositive up to 9 weeks of age when tested with the Tween-20 ELISA. In another study, sows from a naturally infected herd were vaccinated pre-farrowing and their piglets were transferred to isolation facilities at 15 to 17 days of age.38 Maternal antibodies were still detectable by different ELISA tests when some pigs were 15 weeks of age. Comparing four vaccines and using the Tween-20 ELISA test, Thacker et al39 found that some vaccines produced higher titers and more seropositive pigs than others,and that many vaccinated pigs were already seronegative as early as 45 days post vaccination.
The phase of the reproductive cycle may affect serological results. Using an ELISA, Wallgren et al40 showed that antibody levelsof nine sows at farrowing had decreased to 51% of levels detected 4 weeks previously. Two weeks after farrowing, antibody levels had returned to 75% of their initial values. Rautiainen et al41 also found a significant reduction in circulating antibodies prior to farrowing.
Isolation of the organism is the "gold standard" for diagnosis of MH, but it is impractical under field conditions, as special media are required and growth frequently is not detectable for weeks or months.10
Control of M hyopneumoniae pneumonia
Treatment using antimicrobials
Mycoplasma hyopneumoniae is sensitive to several antimicrobials in vitro. Table 1 summarizes the sensitivity results of MH to antimicrobials obtained in four studies.42-45 The minimum inhibitory concentrations (MICs) of tiamulin and several other antimicrobials for MH are low, but for reasons that are not totally clear, enzootic pneumonia does not respond well to treatment.24 Thacker et al46 recently showed that chlortetracycline was effective in preventing lesions and clinical signs associated with MH when administered in the feed prior to infection, but was ineffective or much less effective when administered on days 10 to 24 PI.
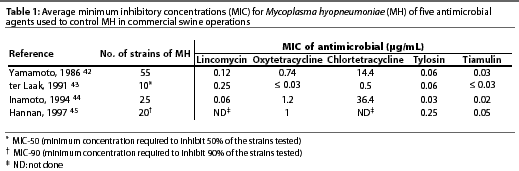
In the face of an outbreak, products with low MICs for MH should be favored (eg, tetracyclines, lincomycin, tiamulin).18 A French study suggests that doxycycline may have value in the treatment of enzootic pneumonia.47 Finally, fluoroquinolones have low MICs for MH and for other respiratory pathogens and have been reported to be effective in treating enzootic pneumonia.24 However, because of the importance of fluoroquinolones in human medicine and the current concerns about antimicrobial resistance, particularly to drugs utilized in both animals and people, the number of countries that will allow or encourage the use of fluoroquinolones in swine will be limited.
Prevention using antimicrobials
Several authors have reported on the efficacy of pulse dosing programs to prevent losses associated with MH infections in grow-finish units.48-50 The products chosen have low MICs for MH (often a combination of tiamulin and a tetracycline, or lincomycin alone or with a tetracycline), and the program schedules vary among researchers. In a recent study, where the goal was to prevent the "18-week wall", Walter et al50 reported good results using tiamulin (38.5 ppm) and chlortetracycline (22 mg per kg body weight) together in the feed for 7 days on weeks 2, 4, 7, 10, and 13 after placement in the finishing unit. Others have recommended strategic medication, where the product used (eg, tetracycline, lincomycin, or tiamulin) is added to the feed for 7 to 10 days, commencing 1 to 3 weeks prior to the anticipated time of disease onset.18
Prevention using vaccination
Vaccination may reduce pneumonia and losses associated with MH.18,24 In severely affected herds, a benefit: cost ratio of up to 5:1 has been achieved.18 Muirhead et al18 have suggested the following criteria for making the decision to vaccinate: presence of MH in the herd; continuous level of respiratory disease; primary or secondary infections associated with PRRS, Apleuropneumoniae, influenza, or pseudorabies; heavy bacterial challenge; necessity for continuous in-feed medication; variable and poor growth associated with respiratory disease; weaning to slaughter mortalities of more than 4%; and cost of vaccination equal to or less than costs of associated mortality and in-feed medication. Clark51 has proposed a simplified general guideline: if a herd is positive for MH and the pigs do not reach 115 kg by 180 days of age, vaccination should be considered.
The scientific literature is not completely clear on vaccination schedules for piglets. Few studies have tested the same vaccine in pigs of different ages within the same farms, and their results do not all show marked performance differences, but there is a tendency for later vaccinations to producebetter results.38,52-55
The time at which pigs are infected with MH may be determined by serology, onset of clinical signs, and PCR, and vaccination may be scheduled accordingly. Sørensen et al12 observed coughing approximately 2 weeks PI, and seroconversion approximately 1 week later. The interval between introduction of naïve pigs into an infected population and onset of clinical signs or seroconversion may vary in field situations.35
In North America, some commercial vaccines have received approval as single-dose products.56,57 In a large US field study, Yeske et al58 showed that one of these vaccinesproduced statistically and economically significant changes in performance when vaccinated pigs were compared to non vaccinated pigs.
The question of whether sows should be vaccinated before farrowing also remains unanswered. A recent study37 showed that piglets with maternal immunity had much less severe lung lesions after challenge than piglets born from naïve sows. However, it was recently reported that piglets of vaccinated sows developed lower antibody titers when vaccinated while they still had maternal antibody titers.38 Two experiments compared the degree of protection to MH challenge when pigs with different levels of maternal immunity were vaccinated.23,38 In the first study, there was no difference in the degree of protection afforded by the vaccine whether pigs were from vaccinated or unvaccinated sows.23 These sows had been vaccinated prior to farrowing, but the herd was free of MH. In the second study, piglets from a naturally infected herd, with high serum antibody levels at the time of vaccination, were not protected as well from challenge at 16 weeks of age as piglets vaccinated later, when maternal antibody levels were lower.38 The authors concluded that when evaluating whether or not maternal antibodies might interfere with activeimmunization, the antibody levels at the time of piglet vaccination may be more important than the age of the pigs.
Although many vaccines may reduce the development of lung lesions after MH challenge under either field or experimental conditions, the mechanism of protection is still unclear. Comparing four different vaccines, Thacker et al39 reported that 'measurements of systemic cellular and humoral immune responses did not necessarily predict the degree of protection against experimental challenge suggesting local, mucosal immunity may be important in protection'. In a more recent study, Thacker et al59 reportedthat vaccination against MH inducedlocal, mucosal, humoral, and cellular immune responses, and reduced the severity of lung lesions in challenged pigs. This study suggested that mucosal antibodies, mediation of the inflammatory response, and cell-mediated immune responsesare important for control of mycoplasmal pneumonia of pigs.
Prevention by non-medical strategies
Non-medical strategies may help to reduce the negative impact of enzootic pneumonia and other respiratory problems. In a recent review, Stärk60 listed the following risk factorsreported to have an impact on respiratory diseases: herd size, air volume, shared airspace, stocking density, diarrhea, sow characteristics, herd type (breeding, fattening), purchase policy, production system(all in-all out, batch, continuous), construction of building or pen, manure handling, feeding technique, access to water,ventilation, draft, bedding, floor, light, heating, hygiene, characteristics of manager, time dependent management factors (weaning, moving), movement of animals, veterinary consultation, temperature, humidity, gases, bioaerosols, dust, season, distance to possibly infected farm, size of neighboring farm, and swine density in the region.
Production of MH-negative pigs from positive herds
It is possible to produce MH-negative pigs from positive herds, particularly when piglets can be weaned early and off-site.61-62 Results from the Deschambault station, an off-site testing station in Canada, where pigs from 20 to 30 different sources are mixed at weaning, offer some interesting insights on this strategy.63 The source herds are of different health status for pathogens, including PRRSV, MH, and Apleuropneumoniae. Ten batches of approximately 450 piglets each have been introduced at an average age of 12.4 days, with excellent performances maintained up to slaughter. Coughing was observed in some batches, but it was believed that six of ten batches were free of MH infection on the basis of clinical signs (absence of coughing), serological results (negative serology in animals tested at the end of the batch), and slaughter inspection data (no cranioventral lesions in lungs). Seroconversion to both MH and PRRSV occurred in three batches, and apart from higher drug cost, performance was comparable to that in other batches. Additional work is required to identify factors responsible for the success of the Deschambault system, and to assess their applicability to commercial multiple site systems where pigs from different sources are mixed and where significant disease problems may occur.
Eradication of MH from
infected herds
Eradication of MH has been described in several countries, including Switzerland, Sweden, and Finland.13-15 The most common technique combines removal from the farm of all animals 10 months of age or younger, a 14-day break in farrowings, and a breeding herd medication program.13 Tiamulin was the most common drug used, but enrofloxacin and lincomycin have also been successful in these programs.13-15
Most studies describing eradication attempts have been conducted in chronically infected herds. Recently, successful eradication programs in herds newly infected with MH were reported from Denmark.64,65 Because of the risk of infection associated with the presence of neighboring infected herds, an eradication program was conducted by Masserey-Wullschleger in Switzerland on an area basis, rather than on a herd basis.66 In two distinct geographic areas, including 345 and 360 farms, an attempt was made to eradicate the organism in a coordinated way from 91 infected farms. A year later, 19 farms were infected with MH, for a reinfection rate of 3.1%. Reinfection was attributed to introduction of MH-infected stock in 53% of cases, and to aerosol transmission in 21% of cases.
Conclusion
Mycoplasma hyopneumoniae has long been present in swine herds. New methods of raising pigs and the upsurge of new pathogens such as PRRSV have complicated the traditional picture that we had of MH and the disease it causes. However, numerous tools are now available to better identify and control the clinical signs and losses associated with MH.
References refereed
1. Ro LH, Ross RF. Comparison of Mycoplasma hyopneumoniae strains by serologic methods. Am J Vet Res. 1983;44:2087-2094.
2. Frey J, Haldimann A, Nicolet J. Chromosomal heterogeneity of various Mycoplasma hyopneumoniae field strains. Int J Syst Bact. 1992;42:275-280.
3. Artiushin S, Minion FC. Arbitrarily primed PCR analysis of Mycoplasma hyopneumoniae field isolates demonstrates genetic heterogeneity. Int J Syst Bact. 1996;46:324-328.
5. Lloyd LC, Etheridge JR. Production of arthritis by intravenous inoculation of Mycoplasma hyopneumoniae: tests on five strains. Res Vet Sci. 1981;30:124-126.
6. Gois M, Kuksa F. Intranasal infection of gnotobiotic piglets with Mycoplasma hyorhinis: differences in virulence of the strains and influence of age on the development of infection. Zbl Vet Med B. 1974;21:352-361.
7. Citti C, Rosengarten R. Mycoplasma genetic variation and its implication for pathogenesis. Wien Klin Wochenschr. 1997;109:562-568.
8. Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Micro Mol Biol Rev. 1998;62:1094-1156.
9. Calsamiglia M, Pijoan C. Colonisation state and colostral immunity to Mycoplasma hyopneumoniae of different parity sows. Vet Rec. 2000;146:530-532.
11. Mattsson JG, Bergström K, Wallgren P, Johansson KE. Detection of Mycoplasma hyopneumoniae in nose swabs from pigs by in vitro amplification of the 16S rRNA gene. J Clin Microb. 1995;33:893-897.
12. Sørensen V, Ahrens P, Barford K, Feenstra AA, Feld NC, Friis NF, Bille-Hansen VB, Jensen NE, Pedersen MW. Mycoplasma hyopneumoniae infection in pigs: Duration of the disease and evaluation of four diagnostic assays. Vet Micr. 1997;54:23-34.
13. Zimmermann W, Odermatt W, Tschudi P. Enzootische pneumoniae (EP): die teilsanierung EP-reinfizierter schweinezuchtbetriebe als alternative zur totalsanierung. Schweiz Arch Tierh. 1989;131:179-186.
14. Wallgren P, Sahlander P, Hasslebäck G, Heldmer E. Control of infections with Mycoplasma hyopneumoniae in swine herds by disrupting the chain of infection, disinfection of buildings and strategic medical treatment. J Vet Med. 1993;40:157-169.
15. Heinonen M, Autio T, Saloniemi H, Tuovinen V. Eradication of Mycoplasma hyopneumoniae from infected swine herds joining the LSO 2000 health
class. Acta Vet Scand. 1999;40:241-252.
16. Stärk KDC. The role of infectious aerosols in disease transmission in pigs. Vet J. 1999;158:164-181.
17. Goodwin RFW. Apparent reinfection of enzootic-pneumonia free pig herds: Search for possible causes. Vet Rec. 1985;116:690-6938.
18. Muirhead, MR., Alexander, TJL. Managing and treating disease in the weaner, grower and finishing periods. In: MR Muirhead et al, eds. Managing pig health and the treatment of disease. Sheffield, UK: 5M Enterprises Ltd. 1997;283-345.
19. Thomsen BL, Jorsal SE, Andersen S, Willeberg P. The Cox regression model applied to risk factor analysis of infections in the breeding and multiplying herds in the Danish SPF system. Prev Vet Med. 1992;12:287-297.
20. Stärk KDC, Keller H, Eggenberger E. Risk factors for the reinfection of specific pathogen-free pig breeding herds with enzootic pneumonia. Vet Rec. 1992;131:532-535.
21. Schulman A, Estola T. Isolation of mycoplasmas from boar semen. Vet Rec. 1974;94:330-331.
22. Mandrup M, Friis NF, Meyling A, Meding JH. Studies on the possible occurrence of mycoplasmas in boar semen. Nord Vet Med. 1975;27:557-561.
24. Ross RF. Mycoplasmal diseases. In: Straw BE, D'Allaire S, Mengeling WL, Taylor DJ. Diseases of Swine. 8th ed. Ames, Iowa: Iowa State University Press; 1999:495-509.
25. Buttenschon J, Friis NF, Aalbaek B, Jensen TK, Iburg T, Mousing J. Microbiology and pathology of fibrinous pericarditis in Danish slaughter pigs. Zentrab Veterinar. 1997;44:271-280.
26. Desrosiers R. Les maladies en émergence chez le porc. Med Vet Que. 1999;29:185-188.
27. Ciprian A, Pijoan C, Cruz T, Camacho J, Tortora J, Colmenares G, Lopez-Revilla R, de la Garza M. Mycoplasma hyopneumoniae increases the susceptibility of pigs to experimental Pasteurella multocida pneumonia. Can J Vet Res. 1988;52:434-438.
28. Amass SF, Clark LK, Van Alstine WG, Bowersock TL, Murphy DA, Knox KE, Albregts SR. Interaction of Mycoplasma hyopneumoniae and Pasteurella multocida infections in swine. JAVMA. 1994;204:102-107.
29. Yagihashi T, Nunoya T, Mitui T, Tajima M. Effect of Mycoplasma hyopneumoniae infection on the development of Haemophilus pleuropneumoniae pneumonia in pigs. Jpn J Vet Sci. 1984;46:705-713.
30. Shibata I, Okada M, Urono K, Samegai Y, Ono M, Sakano T, Sato S. Experimental dual infection of cesarean-derived, colostrum-deprived pigs with Mycoplasma hyopneumoniae and pseudorabies virus. J Vet Med Sci. 1998;60:295-300.
31. Thacker EL, Halbur PG, Ross RF, Thanawongnuwech R, Thacker BJ. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J Clin Microbiol. 1999;37:620-627.
33. Thacker EL, Thacker BJ, Young TF, Halbur PG. Effect of vaccination on the potentiation of porcine reproductive and respiratory syndrome virus (PRRSV)-induced pneumonia by Mycoplasma hyopneumoniae. Vacc. 2000;18:1244-1252.
34. Rautiainen E, Tuovinen V, Levonen K. Monitoring antibodies to Mycoplasma hyopneumoniae in sow colostrum a tool to document freedom of infection. Acta Vet Scand. 2001;41:213-225.
35. Sørensen V, Barfod K, Feld NK, Vraa-Andersen. Application of enzyme-linked immunosorbent assay for the surveillance of Mycoplasma hyopneumoniae infection in pigs. Rev Sci Tech Off Int Epiz. 1993;12:593-604.
40. Wallgren P, Bölske G, Gustafsson S, Mattsson S, Fossum C. Humoral immune responses to Mycoplasma hyopneumoniae in sows and offspring following an outbreak of mycoplasmosis. Vet Microb. 1998;60:193-205.
41. Rautiainen E, Wallgren P. Aspects of the transmission of
protection against Mycoplasma
hyopneumoniae from sow to offspring. J Vet Med B.
2001;48:55-65.
42. Yamamoto K, Koshimizu K, Ogata M. In vitro susceptibility of Mycoplasma hyopneumoniae to antibiotics. Jpn J Vet Sci. 1986;48:1-5.
43. ter Laak EA, Pijpers A, Noordergraaf JH, Schoevers EC, Verheijden JHM. Comparison of methods for in vitro testing of susceptibility of porcine mycoplasma species to antimicrobial agents. Antim Agents Chem. 1991;35:228-233.
44. Inamoto T, Takahashi H, Yamamoto K, Nakai Y, Ogimoto K. Antibiotic susceptibility of Mycoplasma hyopneumoniae isolated from swine. J Vet Med Sci. 1994;56:393-394.
45. Hannan PCT, Windsor GD, de Jong A, Schmeer N, Stegemann M. Comparative susceptibilities of various animal-pathogenic mycoplasmas to fluoroquinolones. Anti Agents Chemo. 1997;41:2037-2040.
47. Bousquet E, Pommier P, Wessel-Robert S, Morvan H, Benoit-Valiergue H, Laval A. Efficacy of doxycycline in feed for the control of pneumonia caused by Pasteurella multocida and Mycoplasma hyopneumoniae in fattening pigs. Vet Rec. 1998;143:269-272.
48. Jouglar JY, Borne PM, Sionneau G, Bardon T, Eclache D. Évaluation de l'intérêt de l'association tiamuline-oxytétracycline dans la prévention des bronchopneumonies du porc charcutier. Rev Med Vet. 1993;144:981-988.
49. Kavanagh NT. The effect of pulse medication with a combination of tiamulin and oxytetracycline on the performance of fattening pigs in a herd infected with enzootic pneumonia. Irish Vet J. 1994;47:58-61.
52. Bilic V, Lipej Z, Valpotic I, Habrun B, Humski A, Njari B. Mycoplasmal pneumonia in pigs in Croatia: first evaluation of a vaccine in fattening pigs. Acta Vet Hung. 1996;44:287-293.
53. Scheidt AB, Mayrose VB, Van Alstine WG, Clark LK, Cline TR, Einstein ME. The effects of vaccinating pigs for mycoplasmal pneumonia in a swine herd affected by enzootic pneumonia. Swine Health Prod. 1994;1:7-11.
59. Thacker EL, Thacker B, Kuhn M, Hawkins PA, Waters WR. Evaluation of local and systemic immune responses induced by intramuscular injection of a Mycoplasma hyopneumoniae bacterin to pigs. Amer J Vet Res. 2000;61:1384-1389.
60. Stärk KDC. Epidemiological investigation of the influence of environmental risk factors on respiratory diseases in swine a literature review. Vet J. 2000;159:37-56.
62. Dritz SS, Chengappa MM, Nelssen JL, Tokach MD, Goodband RD, Nietfeld JC, Staats JJ. Growth and microbial flora of nonmedicated, segregated, early weaned pigs from a commercial swine operation. JAVMA. 1996;208:711-715.
66. Stärk KDC. Veterinary epidemiology a key to sustainable pig production in Switzerland. Schweiz Arch Tierheilk. 2001;143:63-68.
References non refereed
4. Lin BC. Intraspecies differentiation of Mycoplasma hyopneumoniae field strains isolated in the United States. Proc AASP Ann Meet. Nashville, Tennessee. 2001;225-235.
10. Thacker EL. Mycoplasma diagnosis and immunity. Proc AASP Ann Meet. Nashville, Tennessee. 2001;467-469.
23. Thacker BJ, Thacker EL. Influence of maternally-derived antibodies on the efficacy of a Mycoplasma hyopneumoniae bacterin. Proc AASP Ann Meet. Nashville, Tennessee. 2001;513-516.
32. Dufresne L. Diagnosis and control of PRRS in PRDC. Proc AASP Ann Meet. Nashville, Tennessee. 2001;491-496.
36. Andreasen M, Nielsen JP, Baekbo P. Colostral antibodies and duration of maternal immunity: Mycoplasma hyopneumoniae and Actinobacillus pleuropneumoniae serotype 2. Proc IPVS. 2000;446.
37. Thacker B, Thacker E, Halbur P, Minion F, Young T, Erickson B, Thanawongnuwech. The influence of maternally-derived antibodies on Mycoplasma hyopneumoniae infections. Proc IPVS. 2000;454.
38. Jayappa H, Davis R, Rapp-Gabrielsen V, Wasmoen T, Thacker E. Evaluation of the efficacy of Mycoplasma hyopneumoniae bacterin following immunization of young pigs in the presence of varying levels of maternal antibodies. Proc AASV Ann Meet. Nashville, Tennessee. 2001;237-241.
46. Thacker E, Thacker B, Wolff T. Efficacy of Aureomycin chlortetracycline against experimental Mycoplasma hyopneumoniae challenge. Proc IPVS. 2000;457.
51. Clark KL. Mycoplasmal pneumonia control strategies. Proc AD Leman Swine Conf. 2000;87-91.
54. Moore C, Daigneault J. The effect of timing of vaccination with RespiSure to prevent lung lesions in pigs in presence of a high or low challenge of Mycoplasma hyopneumoniae. Proc IPVS 2000;495.
55. Wallgren P, Vallgarda J, Lindberg M, Eliasson-Selling L, Segall T. The efficacy of different vaccination strategies against Mycoplasma hyopneumoniae. Proc IPVS. 2000;461.
56. Roof MB, Burkhart K, Zuckermann FA. Evaluation of the immune response and efficacy of 1 and 2 dose commercial Mycoplasma hyopneumoniae bacterins. Proc AASP Ann Meet. Nashville, Tennessee. 2001;163-167.
57. Keich RL, Sabbadini L, Thacker EL, Thacker BJ, Jolie R, Yancey Jr RJ, McGavin D. Evaluation of
the duration of immunity of RespiSure-ONE following experimental challenge with Mycoplasma hyopneumoniae. Proc AASP Ann Meet. Nashville, Tennessee. 2001;91-93.
58. Yeske P, Garloff C, Kolb JR. Using Ingelvac M. hyo to control mycoplasmal pneumonia in a three site system. Proc IPVS. 2000;468.
63. Broes A, Boutin R, Ethier R. Performances of commingled segregated early weaning pigs in a testing station. Proc IPVS. 2000;329.
64. Lorenzen JB. Eradication of Mycoplasma hyopneumoniae from an acutely infected Danish 2-site 390 sow herd without restocking. Proc IPVS. 2000;340.
65. Damgaard K, Larsen LP, Larsen K, Jensen BP, Szancer J. Eradication of Mycoplasma hyopneumoniae in two newly infected herds. Proc IPVS. 2000;339.
