Diagnostic notes
Non refereed
Update on foot-and-mouth disease in swine
Corrie Brown, DVM, PhD, Dipl ACVP
Department of Veterinary Pathology, College of Veterinary
Medicine, University of
Georgia, Athens, GA 30602-7388; Tel: 706-542-5842; Fax: 706-542-5828;
E-mail: corbrown@vet.uga.edu
Brown C. Update on foot-and-mouth disease in swine. J Swine Health Prod. 2001;9(5):239-242. Also available as a PDF
This is the first article in a series describing foreign animal diseases (FAD) that affect swine and a variety of other farm animals. Although these diseases may never be seen in your practice, the goal of the series is to broaden the swine veterinarian's differential considerations and hopefully enhance early detection of any FAD incursion.
-- David H. Zeman,
Diagnostic Notes Editor
Foot-and-mouth disease (FMD) is a highly contagious viral disease of all cloven-hoofed animals, characterized by fever and vesicle formation in the mouth and on the feet. Economically, it is the most important disease of animals in the world.1 Period. Presence of this disease in a national herd destroys all export possibilities and hinders production so severely that profits for the domestic market evaporate. The disease itself has a low mortality rate but an incredibly high morbidity rate. Affected animals lose production for 2 to 3 weeks, and because of the highly contagious nature of the disease, all animals in a herd are infected. In industrialized systems of agriculture, this short period of poor growth is all it takes to wipe out profits, and countries with significant agricultural exports expend tremendous efforts to keep their national herds free of this virus. Once the virus is known to be present, export markets drop to zero, and without exports, domestic markets soon become glutted and worthless. Foot-and-mouth disease is an exceptionally contagious infection, capable of almost uncontrollable spread.1 Infected animals exhale large quantities of virus, which can then be carried as effective aerosols to neighboring animals and premises. Consequently, it is extremely difficult to control. It is estimated that an FMD outbreak in the US could cost $27 billion in lost trade and markets.2
Etiology and host range
Foot-and-mouth disease virus (FMDV) is a member of the genus Aphthovirus in the family Picornaviridae, all single-stranded, nonenveloped, positive sense RNA viruses. Foot-and-mouth disease was the first animal disease to be recognized as caused by a non-filterable agent, ie, a virus, in 1898.3 Seven serotypes of FMDV have been identified (A, O, C, SAT1, SAT2, SAT3 and Asia1) and are distributed in different geographic regions of the world, although with recent spread, these geographic distinctions are becoming blurred.4 All cloven-hoofed animals are susceptible to infection with FMDV. Experimental and (or) natural infection has been recorded in a number of other species, including elephant, capybara, hedgehog, armadillo, and mouse.4 The vast majority of FMDV strains have demonstrated capability of infecting a very wide host range. However, in Taiwan in 1997, when a vesicular disease in pigs emerged, FMD was initially discounted because cattle in adjacent areas were not diseased. When the diagnosis was finally made and the virus isolated, it turned out to be a "porcinophilic" strain, with strict host preference for pigs.5
Pathogenesis
Infection begins when inhaled viruses reach the lungs of susceptible animals. After a period of replication in the bronchioles, viremia ensues. Viremia is accompanied by fever, and within 1 to 2 days, the virus is established at multiple epithelial sites. The virus replicates in rafts of cells in the stratum spinosum. The infection is cytolytic, with resulting cavity formation within the stratum spinosum. Fluid-laden vesicles are seen grossly at coronary bands, interdigital clefts, tongue, palate, snout, and, in lactating animals, teats. It is thought that the virus is transported into the stratum spinosum via Langerhans cells and that the infected "raft" of cells comprises all the keratinoyctes contacted by one infected Langerhans cell.6
Clinical features
The first signs of clinical disease are fever and reluctance to feed or move about. Closer examination may reveal blanching at the coronary bands as vesicles are forming. Tongue and snout may also show vesicles developing (Figure 1). The vesicles start as small blisters, but then coalesce, producing bullae that rupture easily, leaving an ulcerated epithelial surface. Lameness is usually prominent in pigs, as extensive erosions or ulcerations may develop around the coronary band, occasionally resulting in sloughing of the entire claw (Figure 2). Lesions on the tongue heal rapidly through re-epithelialization, but lesions on the feet tend to become complicated through secondary infection, delaying the healing process. Lactating sows may develop crusting erosions and ulcerations on the teats and are understandably reluctant to nurse their young (Figure 3). The disease is rarely fatal except in very young animals, where it may infect myocardial cells, causing acute myocardial necrosis and heart failure.
Figure 1: Pig with fully-developed snout vesicle and blanched coronary bands, indicating early vesicle formation, 48 hours after experimental infection.
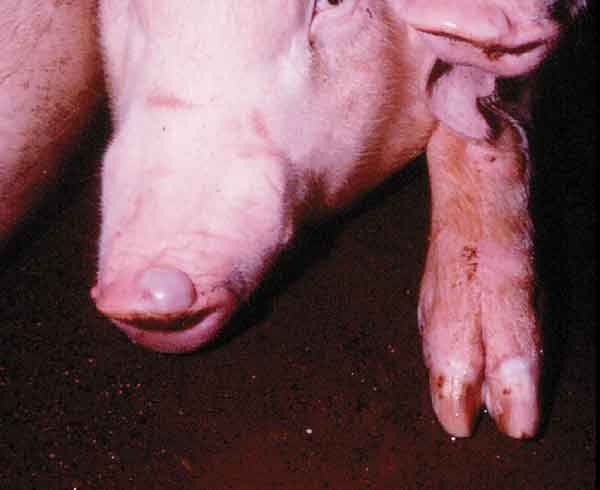
Figure 2: Severe erosions and ulcerations around the coronary band of a pig, with early sloughing of the claw, 96 hours after infection. Photo courtesy of Plum Island collection, Douglas Gregg.
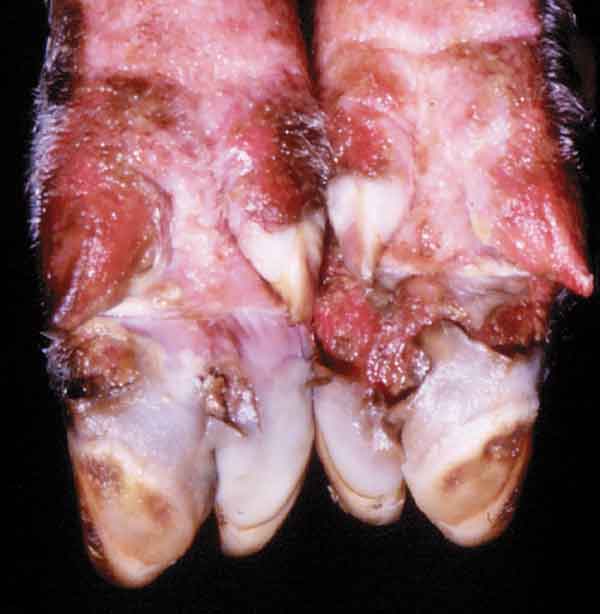
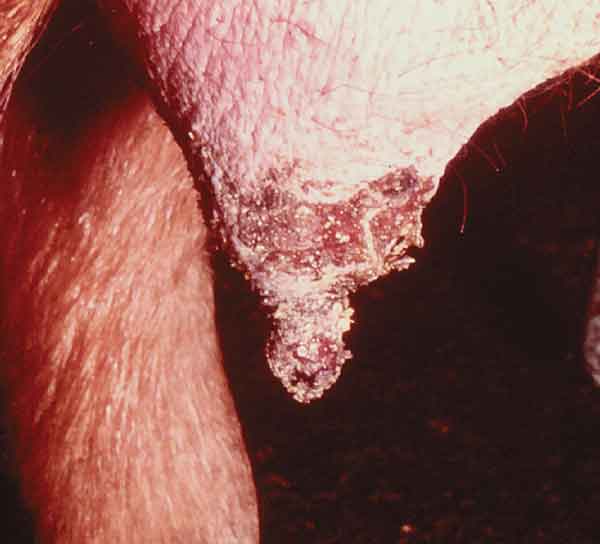
Figure 3: Ulceration of teat epithelium, lactating sow. Photo courtesy of Plum Island collection.
The disease is usually quite noticeable in cattle because of excess salivation and lameness. In sheep and goats, the disease assumes a much less severe course and may even be missed clinically, with subsequent movement of apparently healthy but infected animals into new susceptible areas.7 In addition, all of the cloven-hoofed wildlife, including peccary, deer, bison, antelope, and elk, are susceptible to infection with FMDV.4
Diagnosis
It is not possible to diagnose FMD solely on the basis of the gross or histologic appearance of the disease. Grossly, all diseases causing oral, snout, pedal, or teat erosions should be considered in the differential diagnosis of FMD. Histologically, all vesicular diseases (FMD, swine vesicular disease, vesicular stomatitis) have similar morphologic characteristics and even in the vesicular stage cannot be differentiated from one another microscopically. All are characterized by intra- and intercellular edema in large groups of cells within the stratum spongiosum. Once the vesicles have ruptured, the histology of vesicular diseases is very similar to many erosive and ulcerative diseases.
Laboratory confirmation of an outbreak is essential. In most countries, the appearance of any vesicular disease must be reported to the authorities for adequate investigation. Veterinarians trained by the USDA are responsible for inspecting clinically affected animals, collecting samples, and sending these samples to federal laboratories for diagnosis.
Because of the regulatory implications of the presence of FMD, all laboratory testing is done by the federal government at the Foreign Animal Disease Diagnostic Laboratory, Plum Island. A number of laboratory tests are used for detection of FMDV. In acutely infected animals, ideal materials to collect include vesicular fluid within intact vesicles, or epithelial tags surrounding ruptured vesicles. An ELISA antigen assay may be used for rapid detection of FMDV if there is sufficient virus in the sample. If the sample is inadequate or the test results are inconclusive, cell cultures are inoculated. When cytopathic effects are detected in cell culture, the fluids are tested by ELISA. Virus neutralization and ELISA are used to test for serum antibodies: both of these serological tests are serotype specific.8
Transmission and spread of outbreaks
Under natural conditions, the most common form of transmission is by aerosol, with high concentrations of infectious particles exhaled by an animal in the acute phase of the disease being carried on the air to the respiratory tract of a susceptible animal. More than any other infected species, pigs produce enormous quantities of virus, with over a hundred million infectious virus particles exhaled per day. Consequently, pigs are often referred to as the "amplifier hosts" of FMD. The virus is also present in vesicular fluid and saliva, and at the peak of infection can be found in blood and tissues of the affected animal.4 Although the virus is readily inactivated in muscle under post mortem conditions because of the rapid drop in pH, it may survive in pockets of lymphoid tissue and bone marrow.
Most new outbreaks begin with animal contact or consumption of animal byproducts. Illegally imported, virus-contaminated meat products fed as garbage to pigs have caused many new outbreaks in the world, and are suspected as the cause of the epidemic in eastern Russia in 20009 and in the UK in 2001.10 Although most countries require that garbage fed to pigs be thoroughly cooked, which inactivates the virus, not all garbage-feeding operations adhere to this practice. Introduction of healthy carrier animals has also been responsible for sparking outbreaks. Recovered pigs clear the infection completely, but in ruminant species, the virus may be harbored in the oropharynx of a percentage of recovered animals, which become healthy carriers, with potential spread to new areas.11 Also, infected sheep and goats, which often show very few clinical signs of the disease, may be moved to new areas, generating an outbreak such as the one that occurred in Greece in 1995.7 The virus survives in the environment fairly well and can persist for up to 1 month under the favorable conditions of high humidity, cool temperature, and appropriate pH (>6.0 and <9.0). Transport of contaminated hay into the Republic of Korea and Japan is thought to be the cause of the outbreaks there in 2000.9 In addition, veterinary equipment and vehicles are notorious for acting as fomites to carry FMDV from one premise to another. People coming to and going from an infected farm can also act as fomites, carrying the virus on their clothes or shoes. It has been shown that FMDV can reside passively in the human nasal passages for 24 hours and so be carried to new areas.12
Control
Control in an outbreak requires rapid and effective action. The USDA maintains emergency disease guidelines for all foreign animal diseases, including FMD.13 In an outbreak, the amount of economic damage and the number of animals that will have to be destroyed are directly proportional to the length of time the disease is present prior to being accurately diagnosed. All infected and in-contact animals must be isolated and destroyed, as they are producing, or about to produce, large amounts of virus aerosols.14 Monitoring through serosurveillance must be started immediately. Movement of humans, equipment, all materials including garbage, and non-susceptible animals must also be closely monitored, and materials and equipment disinfected regularly. It is crucial to spray tires and the undersides of all vehicles leaving an infected area. Effective disinfectants include 2% acetic acid (half-strength kitchen vinegar) or 5.25% sodium hypochlorite (3 parts laundry bleach, 2 parts water). If the initial focus is rapidly identified before any significant spread has occurred, ring vaccination is employed to create a "firewall" to keep the virus within a localized zone.15 Only killed-virus, serotype-specific vaccines are available. Immunity from vaccination lasts only 6 months. A vaccinated animal could be infected with a second serotype and become clinically ill or could be infected with the same serotype and experience no clinical illness, but might be an effective shedder of the virus.16 Once the outbreak has been contained, vaccinated animals are usually euthanized.
Current threat
Currently, the world is experiencing a "global pandemic" of FMD. In the year 2000, the virus made significant incursions into many areas of Asia and South America, some of these areas having been free of the disease for decades. Then, in 2001, the pandemic moved into Europe and was rekindled in South America. The current nature of international trade mandates that there will be ever increasing amounts of traffic of people, animals, and animal products. Many of the countries experiencing outbreaks in the past 2 years are major trading partners of the US. Although border inspections and import controls have tightened considerably, the very real possibility of FMDV entering the US is greater than ever. It is probably no longer a question of "if" but "when" FMD strikes the US. Our only realistic hope of controlling a domestic incursion will be to diagnose it at the very earliest possible moment. Consequently, awareness and education are of the utmost importance.
And, remember, there are two ways to become famous when FMD
enters the USA -- to recognize it OR
to miss it!!!
References -- refereed
2. Brown C, Slenning B. Impact and risk of foreign animal diseases. J Am Vet Med Assoc. 1996;208:1038-1040
4. House J, Mebus C. Foot-and-mouth disease. In: Buisch W, Hyde J, Mebus C, eds. Foreign Animal Diseases. 6th ed. Richmond, Virginia: United States Animal Health Association; 1998:213-224.
5. Knowles N, Davies P, Henry T, O'Donnell V, Pacheco J, Mason P. Emergence in Asia of foot-and-mouth disease viruses with altered host range: characterization of alterations in the 3A protein. J Virol. 2001;75:1551-1556.
6. Brown C, Olander H, Meyer R. Pathogenesis of foot-and-mouth disease in swine, studied by in-situ hybridization. J Comp Pathol. 1995;113:51-58.
7. Barnett P, Cox S. The role of small ruminants in the epidemiology and transmission of foot-and-mouth disease. Vet J. 1999;158:6-13.
11. Bergmann I, Malirat V, Auge de Mello P, Gomes I. Detection of foot-and-mouth disease viral sequences in various fluids and tissues during persistence of the virus in cattle. Am J Vet Res. 1996;57:134-137.
12. Sellers RF, Herniman KA, Mann JA. Transfer of foot-and-mouth disease virus in the nose of man from infected to non-infected animals. Vet Rec. 1971;89:447-9.
14. Ferguson N, Donnelly C, Anderson R. The foot-and-mouth epidemic in Great Britain: Pattern of spread and impact of interventions. Science. 2001;292:1155-1160.
15. Garland A. Vital elements for the successful control of foot-and-mouth disease by vaccination. Vaccine 1999;17:1760-1766.
16. Sellers R, Herniman K, Gumm I. The airborne dispersal of foot-and-mouth disease virus from vaccinated and recovered pigs, cattle and sheep after exposure to infection. Res Vet Sci. 1977;23:70-75.
References -- non refereed
1. Brown C. Economic considerations of agricultural diseases. In: Frazier T, Richardson D, eds. Food and agricultural security: guarding against natural threats and terrorist attacks affecting health, national food supplies, and agricultural economics. Proceedings of an international conference. Washington DC, USA. Ann NY Acad Sci. 1999;894:92-94.
3. Rueckert R. Picornaviridae: The viruses and their replication. In: Fields B, Knipe D, Howley P, et al, eds. Field's Virology. 3rd ed. Philadelphia: Lippincott-Raven Publishers; 1996:609-782
8. Donaldson A, Kitching P, Barnett P. Foot-and-mouth disease. In: Manual of Standards for Diagnostic Tests and Vaccines. Paris, France: Office International des Epizooties; 1996:47-56.
9. Office International des Epizooties Press Release. OIE emergency meeting on foot and mouth disease in East Asia. http://www.oie.int/eng/press/A_000622.htm. Accessed July 18, 2001.
10. Source of the outbreak. Department for Environment, Food &Rural Affairs, 1 May 2001. www.maff.gov.uk/animalh/diseases/fmd/about/current/source.asp . Accessed July 20, 2001.
13. Foot-and-Mouth Disease Emergency Disease Guidelines. Riverdale, Maryland: US Dept of Agriculture, Animal and Plant Health Inspection Service, Veterinary Services; 2001.

